Food Insecurity is a Leading Health-Related Social Need Among Patients With Diabetes
Pharmacy Times
MAY 2, 2023
Screening tools are becoming widely used to help clinicians address the needs of patients with diabetes to improve health outcomes.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmacy Times
MAY 2, 2023
Screening tools are becoming widely used to help clinicians address the needs of patients with diabetes to improve health outcomes.

World of DTC Marketing
MARCH 23, 2022
Do patients care? The program was codified into law under the Food and Drug Safety and Innovation Act (FDASIA) in 2012. Do patients care? If you’re a cancer patient and your oncologist is prescribing a drug approved via accelerated Approval, are you going to refuse treatment? The answer to that would be “no.”
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

World of DTC Marketing
MARCH 21, 2021
Patient-centric has become buzzwords within the industry. It should be patient-centric if there an ROI. There are many different types of cancer and how they destroy the body differs by patient populations. It’s just too damn easy to take a pill rather than give up bad foods or exercise.

World of DTC Marketing
AUGUST 22, 2022
Globally, nearly half of deaths due to cancer can be attributable to preventable risk factors, including three leading risks of smoking, drinking too much alcohol, or having a high body mass index, a new paper suggests. The costs associated with direct cancer care are staggering for patients and their families. health care spending.

Pharmaceutical Technology
OCTOBER 11, 2022
Swiss biotech company Stalicla has signed a licensing deal with Evgen Pharma for the latter’s lead asset, SFX-01, in neurodevelopmental disorders and schizophrenia. in milestone payments that include $5m upon receipt of the investigational new drug (IND) from the US Food and Drug Administration (FDA), which is expected late next year.

Pharmaceutical Technology
APRIL 12, 2023
Pharming Group has conducted the first commercial shipments of oral selective PI3Kδ inhibitor Joenja (leniolisib) to patients diagnosed with activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS) in the US. In March 2023, Joenja secured approval from the US Food and Drug Administration (FDA) to treat the targeted patients.

Pharmaceutical Technology
JUNE 8, 2023
The use of 4D Molecular Therapeutics’ (4DMT) aerosolised gene therapy 4D-710 has improved the quality-of-life and spirometry-measured outcomes in three cystic fibrosis patients , based on early results from a Phase I/II study presented at this year’s annual meeting of the European Cystic Fibrosis Society (ECFS).

MedCity News
JUNE 8, 2023
Situations like these can be an annoyance for neurotypical patients. For neurodivergent patients, they’re obstacles to getting adequate care. Eighty-five percent of medical students feel inadequately prepared to provide care for autistic patients. Sitting on the rough, crinkly paper on the exam table. Take autism, for example.

European Pharmaceutical Review
MARCH 8, 2023
Now working as a woman in pharma, Dr Ekaterina Malievskaia highlighted her female inspirations in the industry, two of which have held leading roles at Bristol Myers Squibb and the US Food and Drug Administration (FDA). I used to prescribe antidepressants for patients but never considered psychiatry as a profession.
European Pharmaceutical Review
OCTOBER 21, 2022
Clinical trial results presented at the US IDWeek conference, revealed a three-dose series of the HEPLISAV-B vaccine prevents hepatitis B virus (HBV) in HIV patients not previously vaccinated against or infected with the virus. A breakdown of the data showed: HbsAb levels were greater than 1000mIU/ml in 88 percent of patients.
Pharmaceutical Technology
MAY 15, 2023
Innovation S-curve for the pharmaceutical industry Periodontitis drugs is a notable innovation area in the pharmaceutical industry Periodontal diseases, which may lead to the damage of teeth or bone, are caused by infections and inflammation of the gums and bone that surround and support the teeth.

Clarivate
NOVEMBER 13, 2023
Clinical outcome assessments can take years to generate but may pay big dividends in patient-focused drug development, centering the patient experience and potentially bolstering a product’s case with regulators and payers. A clinical outcome assessment is a measure that describes or reflects how a patient feels, functions or survives.
European Pharmaceutical Review
MARCH 18, 2024
A Phase III trial has shown that compared oral drug delivery, administering levodopa through an infusion pump led to nearly two hours of day (1.72) of additional time in which the medicine reduced symptoms in Parkinson’s patients. There were 381 Parkinson’s patients enrolled in the trial. and Joan A.
European Pharmaceutical Review
AUGUST 11, 2022
Hospitalised patients on high-flow oxygen for severe COVID-19 pneumonia were nearly 50 percent less likely to die when treated with antiviral remdesivir in combination with steroid dexamethasone and baricitinib, according to research from Rutgers Ernest Mario School of Pharmacy in the US.

pharmaphorum
JUNE 27, 2022
7008), which allows manufacturers to share vital information with healthcare payers and plans while treatments are pending Food and Drug Administration (FDA) approval, was passed by the House of Representatives and is awaiting approval in the Senate. Affecting patient care. The AMCP backed bill (H.R. “Also, the entire bill, H.R.

European Pharmaceutical Review
APRIL 17, 2024
Roche’s twice-yearly, 10-minute subcutaneous injection of OCREVUS ® (ocrelizumab) has shown significant promise for patients with either with relapsing or primary progressive multiple sclerosis (RMS or PPMS). percent had no relapse) in patients through 48 weeks of the treatment.
European Pharmaceutical Review
MAY 12, 2023
Innovative therapies for rare diseases The acquisition will help to drive the growth of CTI’s lead product, kinase inhibitor VONJO ® (pacritinib) “in treating myeloproliferative disease,” stated Dr Adam Craig, President, Chief Executive Officer and Interim Chief Medical Officer of CTI BioPharma.

Pharma Marketing Network
MARCH 3, 2021
But what about patients? And what about the disrupted patient journey? What can our industry do to ensure patients maintain continuity of care at a time when good health is imperative? Reassess the Patient Journey. Enhance Patient Services Programs. Here, we offer four ways pharma marketers can help.

Clarivate
NOVEMBER 10, 2022
Clinical outcome assessments can take years to generate but may pay big dividends in patient-focused drug development, centering the patient experience and potentially bolstering a product’s case with regulators and payers. Patient focused drug development in alopecia areata clinical trials.

World of DTC Marketing
DECEMBER 2, 2021
” So, are we supposed to believe that better images in DTC ads lead to patients asking for an Rx? Ad recall is essential for CPGs, but for prescription drugs, the question that’s missing is “what action did it lead to? Higher numeracy was associated with better gist and verbatim recall.”
European Pharmaceutical Review
DECEMBER 5, 2023
Efficacy of the treatment was compared to placebo in patients with lower risk myelodysplastic syndromes (MDS) relapsed/refractory or ineligible for erythropoiesis stimulating agents (ESAs). The promise of telomerase inhibitors for treating blood cancer Trial results The clinical trial enrolled a total of 178 patients. g/dL for placebo.
Pharmaceutical Technology
JUNE 8, 2023
The US Food Drug Administration (FDA) has granted orphan drug designation to DTx Pharma’s investigational DTx-1252 for the treatment of Charcot-Marie-Tooth disease Type 1A (CMT1A). CMT1A is a progressive neuromuscular autosomal-dominant disease that leads to life-long loss of muscle function, as well as disability.

European Pharmaceutical Review
DECEMBER 5, 2023
Incretins are “gut hormones that are secreted after food intake and play a role in modulating blood glucose by stimulating insulin secretion and suppressing appetite,” Roche highlighted. Roche has agreed to acquire biotech Carmot Therapeutics for an upfront purchase price of $2.7
Pharmaceutical Technology
JUNE 6, 2023
Findings from a two-year placebo-controlled, double-blind Phase II trial showed that Stargardt patients treated with gildeuretinol once a day as a pill experienced a statistically and clinically meaningful slowing of retinal damage over placebo. Additional clinical data show halting of disease in patients, including children.”

Legacy MEDSearch
JULY 21, 2023
UroMems , a global company developing innovative, mechatronics technology to treat stress urinary incontinence (SUI), announced today that it has successfully completed the first-ever implant of the UroActive smart, automated artificial urinary sphincter (AUS) in a female patient. Food and Drug Administration).
World of DTC Marketing
AUGUST 31, 2022
“Obesity is a leading cause of preventable disease and death among U.S. Foods lacking in the Western diet-whole grains, vegetables, fruits, and nuts-seem to help with weight control and also help prevent chronic disease. and see the gigantic platters of food in front of them. at the National Institutes of Health.
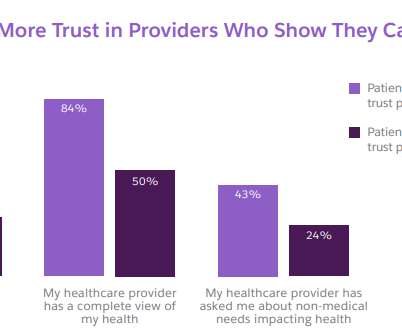
Salesforce
SEPTEMBER 16, 2022
Two patients with the same diagnosis, prescribed the same medical regime, and treated by the same doctor in the same hospital could have wildly different outcomes. Examining your patients’ health within the context of their lives is critical. Examining your patients’ health within the context of their lives is critical.

Pharmaceutical Technology
JUNE 21, 2023
AGEPHA Pharma has received approval from the US Food and Drug Administration (FDA) for LODOCO (colchicine, 0.5 The regulatory approval was based on the findings obtained from a double-blind, multinational, placebo-controlled, randomised clinical trial conducted in 5,522 chronic coronary disease patients.

European Pharmaceutical Review
NOVEMBER 28, 2023
The US Food and Drug Administration (FDA) has approved the first drug for desmoid tumours (desmoid fibromatosis), an oral gamma secretase inhibitor. According to the FDA, this clinical trial evaluated 142 adult patients with progressing desmoid tumours not suitable to surgery.

Pharmaceutical Technology
APRIL 18, 2023
IntelGenx Corp announced that the US Food and Drug Administration (FDA) has approved the company’s Rizafilm VersaFilm new drug application (NDA) for the treatment of acute migraine. It dissolves rapidly releasing the active ingredient in the mouth, meaning there is no requirement for the patient to swallow a pill.

European Pharmaceutical Review
JULY 5, 2022
Several lifestyle factors have been shown to increase the risk of developing stomach cancer, including alcohol consumption, smoking and consuming foods preserved by salts. Several non-clinical and clinical studies provide proof of concept for metronomic dosing, including improved patient outcomes. New hope for stomach cancer patients.
European Pharmaceutical Review
JULY 18, 2022
In children common symptoms include acid reflux, vomiting, abdominal discomfort, trouble swallowing and a failure to thrive; these impact growth and development – and can cause food-related fear and anxiety which can persist through adulthood. There are currently no approved treatments for children with EoE under 12 years of age.

Legacy MEDSearch
SEPTEMBER 12, 2023
Food and Drug Administration (FDA) has approved the LimFlow System to help people with CLTI who have no other suitable endovascular or surgical treatment options and are facing major amputation. At LimFlow, our vision is to achieve great outcomes for patients suffering from CLTI.
Pharma Leaders
MARCH 20, 2023
The US Food and Drug Administration (FDA) has granted fast track designation to Arrowhead Pharmaceuticals’ ARO-APOC3 that helps to lower triglycerides in adult patients with familial chylomicronemia syndrome (FCS). The Phase 3 PALISADE clinical study (NCT05089084) is investigating ARO-APOC3 in patients with FCS.
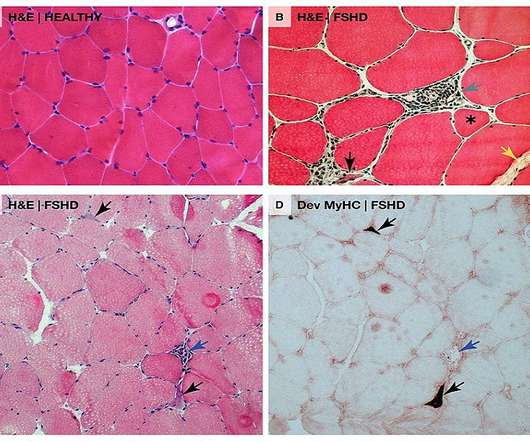
Pharmaceutical Technology
JANUARY 19, 2023
The US Food and Drug Administration (FDA) has granted Fast Track designation for Avidity Biosciences’ AOC 1020 to treat facioscapulohumeral muscular dystrophy (FSHD). This DUX4 protein abnormal expression leads to modifications in gene expression in muscle cells which are associated with progressive muscle function loss in FSHD patients.

World of DTC Marketing
FEBRUARY 16, 2022
The statistics are alarming, especially for a country that spends so much on healthcare, but we continue to ignore patients who are obese. Pfizer has made billions of dollars from their COVID vaccine but shouldn’t they have a responsibility to warn people that obesity is a leading cause of severe COVID? What about insurers?

World of DTC Marketing
NOVEMBER 1, 2020
Fauci, the country’s leading infectious-disease expert, said in a wide-ranging interview late Friday. Fauci, a leading member of the government’s coronavirus response, said the United States needed to make an “abrupt change” in public health practices and behaviors. We’re in for a whole lot of hurt.

World of DTC Marketing
FEBRUARY 10, 2022
30, 2021, the study’s lead author, Thomas J. It isn’t real food; it is the packaged, highly refined, chemically-laden products marketed to us as “food” that is killing us and will continue to do so until we reframe what is being perpetrated on consumers in terms of addiction. . 1, 2020, and Sept.
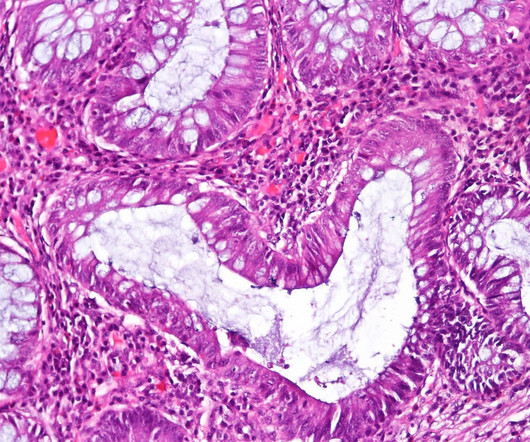
Pharmaceutical Technology
APRIL 3, 2023
It also decreases the number of injections needed by patients who require a dose of 80 mg/mL or above. Sandoz Europe Region head Rebecca Guntern said: “Living with a chronic disease can take a significant toll on a patient’s quality of life.

European Pharmaceutical Review
JULY 28, 2023
Publication of results of the Phase III SKYLARK study “is a pinnacle moment in treating postpartum depression ( PDD ),” stated Dr Kristina M Deligiannidis, Professor at the Institute of Behavioral Science at the Feinstein Institutes, the trial’s principal investigator, lead author of the paper.

Legacy MEDSearch
APRIL 7, 2023
Food and Drug Administration (FDA) has granted 510(k) clearance to the Axiom PSR System for use with the company’s Kinos Axiom Total Ankle System. The Axiom PSR metal resection guides sit noticeably better on the bone and offer greater saw control compared to the historically polymer patient-specific instruments.

European Pharmaceutical Review
DECEMBER 22, 2023
The deal includes Karuna’s lead asset KarXT (xanomeline-trospium), a potential first-in-class treatment for schizophrenia. Currently under review by the US Food and Drug Administration (FDA), KarXT is expected to launch in the US in late 2024. per share in cash.
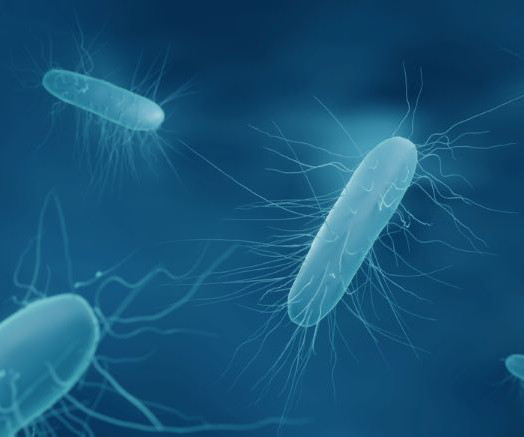
European Pharmaceutical Review
OCTOBER 2, 2023
has discontinued the Phase IIb clinical trial of its lead antibiotic candidate, ibezapolstat, for Clostridioides difficile infection (CDI). In the finished Phase IIa segment of this trial, 10 patients with diarrhoea caused by C. Every patient was investigated for recurrence for 28± 2 days. Developing a front-line C.

Pharma Marketing Network
MARCH 3, 2021
But what about patients? And what about the disrupted patient journey? What can our industry do to ensure patients maintain continuity of care at a time when good health is imperative? Reassess the Patient Journey. Enhance Patient Services Programs. Here, we offer four ways pharma marketers can help.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content