TOP 10 CHALLENGES IN PHARMACEUTICAL PRODUCT LIFE CYCLE MANAGEMENT
eMediWrite
OCTOBER 3, 2022
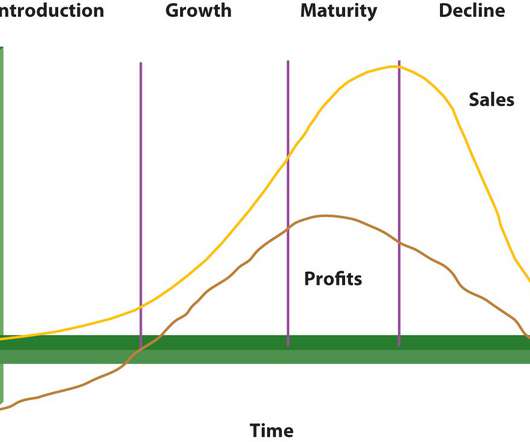
Understanding a product’s life cycle can assist a company in determining its position in the market relative to rivals and the success or failure of the product. Many pharmaceutical firms find it necessary to put additional quality control systems in place since they were responsible for producing a high-quality products.
































Let's personalize your content