Rapid pseudomonas aeruginosa detection method developed
European Pharmaceutical Review
MARCH 13, 2024
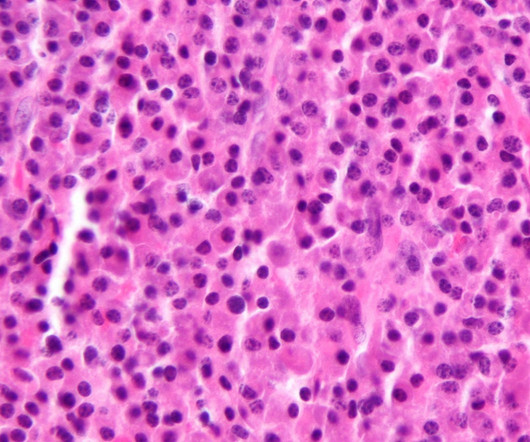
In the paper, the author acknowledged that topical medicinal products require the absence of bacterial species such as P. In the control bottle, the colour stayed green,” Puscas explained. For example, the author shared that the procedure can be used in microbiological laboratories of various levels. aeruginosa.












































Let's personalize your content