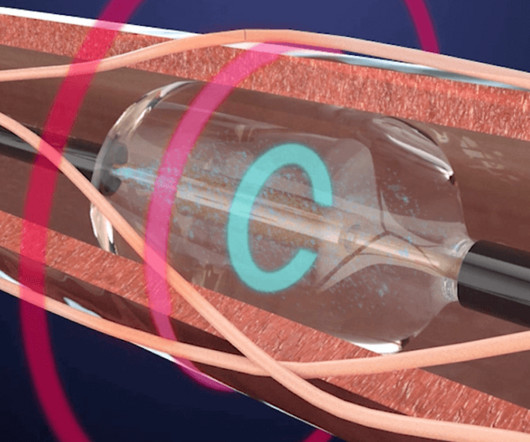
ReCor Medical Announces Two Concurrent JAMA Network Publications of Study Results on the Paradise Ultrasound Renal Denervation System for the Treatment of Hypertension
Legacy MEDSearch
FEBRUARY 28, 2023
In addition, pooled analysis results from the combined primary efficacy endpoint and safety data from RADIANCE SOLO, RADIANCE TRIO, and RADIANCE II were concurrently published in JAMA Cardiology. The study also achieved its primary safety composite outcome with no major adverse events observed. mmHg, compared to a reduction of -1.8






































Let's personalize your content