Axial3D Receives FDA Clearance for Axial3D INSIGHT™ Medical Image Segmentation Platform
Legacy MEDSearch
JULY 31, 2023
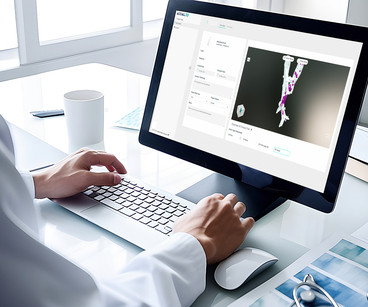
Axial3D, a leader in medical segmentation and 3D solutions, today announced that it is the first to receive FDA clearance for an automated, AI-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic, maxillofacial, and cardiovascular applications.































Let's personalize your content