Apellis scraps pegcetacoplan development in ALS after phase 2 trial flop
Fierce Pharma
MAY 25, 2023
Apellis scraps pegcetacoplan development in ALS after phase 2 trial flop zbecker Thu, 05/25/2023 - 10:42

Fierce Pharma
MAY 25, 2023
Apellis scraps pegcetacoplan development in ALS after phase 2 trial flop zbecker Thu, 05/25/2023 - 10:42

MedCity News
MAY 25, 2023
It’s clear that services like telehealth and remote patient monitoring have potential to provide value within the healthcare industry, but hospitals and digital health companies need to show payers clearer evidence of the outcomes these care modalities can produce in order to earn reimbursement, panelists argued during MedCity’s INVEST conference.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 25, 2023
Johnson & Johnson takes another step in CAR-T race with Bristol Myers in multiple myeloma kdunleavy Thu, 05/25/2023 - 10:52
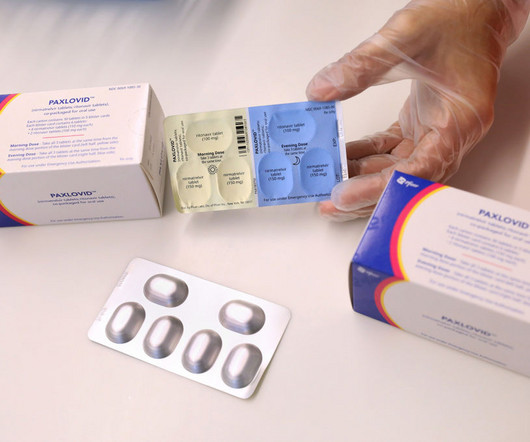
MedCity News
MAY 25, 2023
Paxlovid, a Pfizer antiviral awarded FDA emergency authorization in 2021 for treating Covid-19, is now approved for treating adults. Results from a pivotal clinical showed that Paxlovid led to an 86% reduction in Covid-19-related hospitalization or death from any cause compared to a placebo.

Fierce Pharma
MAY 25, 2023
ASCO preview: Merck's Keytruda, Novartis' Kisqali face their moment of truth in early-stage cancers aliu Thu, 05/25/2023 - 14:31
Pharmaceutical Technology
MAY 25, 2023
Appili Therapeutics has received a US patent for ATI-1501 , a liquid oral reformulation of metronidazole. The patent claims for ATI-1501 were published by the US patent and trademark office under US application no. 18/072,154, filed on 30 November 2022. It covers the composition and preparation methods for ATI-1501 until 2039. Metronidazole is a widely used frontline oral treatment for parasitic and anaerobic bacterial infections.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 25, 2023
Clover Biopharmaceuticals has established a commercial collaboration with Keyuan Xinhai (Beijing) Medical Products Trading (Kyuan Trade) for the launch of its quadrivalent seasonal influenza vaccine, AdimFlu-S (QIS), in China. The China National Medical Products Administration approved AdimFlu-S (QIS) in January 2022 for individuals aged three years and above.

Fierce Pharma
MAY 25, 2023
'The Top Line': The 20 most influential people in biopharma, plus this week's headlines tcarey Thu, 05/25/2023 - 14:21

PM360
MAY 25, 2023
The world is full of tradeoffs—it’s the way progress is made while proportionately managing risk. When it comes to decentralized clinical trials (DCTs), arguably the most transformative innovation clinical research has seen in decades, the same is true. The new Food and Drug Administration (FDA)’s Decentralized Clinical Trial Draft Guidance issued May 1 st has started to outline guidelines for increased oversight and planning while simultaneously supporting broader DCT adoption.

Fierce Pharma
MAY 25, 2023
As drugmakers advance a wave of new cell and gene therapies, contract manufacturers have captured a larger share of the biopharma spotlight in recent years as they push to meet ever-increasing dema | As drugmakers advance a wave of new cell and gene therapies, contract manufacturers have captured a larger share of the biopharma spotlight in recent years as they push to meet ever-increasing demand.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

PM360
MAY 25, 2023
ChatGPT is poised to revolutionize the pharmaceutical industry by providing innovative communication and marketing strategies. With the assistance of AI tools, pharma marketers can develop personalized sales messages and educational content, improving their engagement with healthcare providers and equipping them to deliver better patient care. ChatGPT’s natural language processing (NLP) technology enables human-like conversations combined with the analysis of vast quantities of data, allowing ph

Pharmaceutical Technology
MAY 25, 2023
ElevateBio has raised $401m in a Series D financing round for advancing its technology platforms to expedite the design, production and development of cell and gene therapies. The technology platforms include the Life Edit gene editing platform, an RNA, cell, protein, vector engineering and induced pluripotent stem cells (iPSCs) platform. ElevateBio intends to use the funds to advance its genetic medicine current good manufacturing practice (cGMP) and process development business, BaseCamp.

MedCity News
MAY 25, 2023
The Medicaid continuous enrollment provision ended March 31, meaning states are resuming the renewal process for Medicaid and disenrolling those who no longer qualify for coverage or don’t renew coverage. Most Medicaid enrollees are unprepared for this, a survey from the Kaiser Family Foundation showed.

PM360
MAY 25, 2023
Mental health continues to be a national crisis that is only becoming more prevalent, especially since the pandemic. According to the National Alliance on Mental Illness (NAMI) , 22.8% of U.S. adults experienced mental illness in 2021, which represents about 1 in 5 adults or 57.8 million people. Furthermore, Trilliant Health found that behavioral health volumes reached 18.1% above pre-pandemic levels by Q2 2022.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

PharmaVoice
MAY 25, 2023
The two institutional battles offer a glimpse into the tension between pharma innovation and regulation that could reverberate across the industry.

PM360
MAY 25, 2023
The pandemic had a huge impact on the way population health decision-makers (PHDMs) navigate our industry, and now that it’s clear many of these shifts are here to stay, pharmaceutical manufacturers need to adjust accordingly. Results from Spherico’s annual PHDM survey point to an evolution in how payers want to communicate with pharmaceutical companies and what they want to talk about.

MedCity News
MAY 25, 2023
Fundraising rounds for health startups are taking longer to close and investors are doing more due diligence, said Ulili Onovakpuri, managing partner of Kapor Capital, during a Monday panel at the MedCity INVEST conference in Chicago.

PM360
MAY 25, 2023
Across Europe, pharmaceutical reps are struggling to get access to healthcare professionals. This problem was amplified by the pandemic but had been growing prior to that. In conjunction with CMI Media Group’s international expansion beyond the United States, we conducted research and published a report in partnership with Medscape which delved into the media habits of physicians across the EU5 countries: France, Germany, Italy, Spain, and the United Kingdom.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
MAY 25, 2023
The competition saw 23 healthcare startups across biopharma, medical devices, and health tech geared for consumers/employers as well as payers/providers pitch their business plans to teams of investor judges.
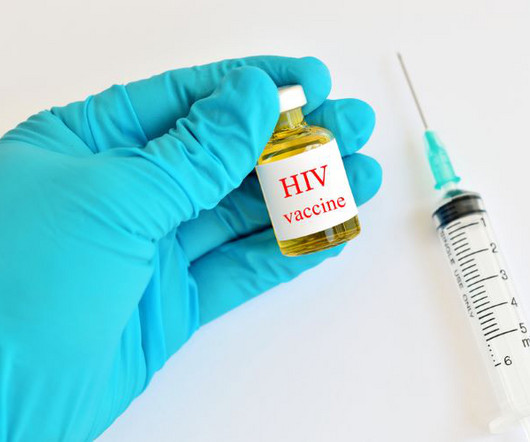
European Pharmaceutical Review
MAY 25, 2023
Researchers have characterised robust T-cell responses in volunteers participating in a Phase I trial for a self-assembling nanoparticle HIV vaccine. The results, published in Science Translational Medicine is a major step in the development of a vaccine for overcoming the HIV/AIDS epidemic. “We were quite impressed that this vaccine candidate produced such a vigorous T-cell response in almost all trial participants who received the vaccine,” explained Dr Julie McElrath, PhD, co-senior author

Pharmaceutical Technology
MAY 25, 2023
A new vaccine developed by the Serum Institute of India to fight meningococcal disease could help eliminate meningitis across Africa. The results from a trial, published in The New England Journal of Medicine , found the vaccine was associated with a strong immune response and good safety profile. The trial, conducted in 1,800 healthy individuals ranging from ages 2-29 years in Mali and The Gambia in 2021, found NmCV-5 induced a strong immune response across five strains of meningococcal bacteri
European Pharmaceutical Review
MAY 25, 2023
CPHI’s Pharmapack Awards 2024 are open for submissions. Two new categories and eight prizes in total are divided into two separate streams: Exhibitor Innovation Awards and the Health Product Awards. The Pharmapack Awards 2024 (January 24-25 2024) will be held during the event’s opening day. CPHI’s awards provide insight into the industry’s latest developments and innovations that will influence global drug delivery and packaging in the year ahead.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

PharmaTimes
MAY 25, 2023
The collaboration aims to develop novel preclinical models with a view to identifying new treatments

PharmExec
MAY 25, 2023
The 2023 Emerging Pharma Leaders share with the Pharmaceutical Executive editors what makes a good leader, what has made them the proudest in their career, lessons they wish they had known when starting out, and more. (This is the video podcast of the Pharm Exec Podcast episode!

PharmaTimes
MAY 25, 2023
Tumour treatment among all phase 1a cohorts in were concluded with no safety concerns

Pharmaceutical Technology
MAY 25, 2023
The Medicines and Healthcare products Regulatory Agency (MHRA) aims to launch a pilot genetic biobank that will gather patient data to associate drug-related adverse events to their genetic makeup. The Yellow Card biobank will launch as a joint venture with the UK-government funded entity Genomics England on June 1. The biobank will work in parallel with the MHRA’s Yellow Card website, which is used for reporting adverse events and side effects caused by medicines and medical devices, per the 25

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Clarify Health
MAY 25, 2023
Value-Based Care (VBC) has been at the forefront of healthcare transformation, with its emphasis on improving patient outcomes and reducing costs. However, achieving these dual goals necessitates innovation in physician incentives. Boost clinical effectiveness with targeted physician incentives A new type of physician incentive called micro-incentives have emerged as a promising model for driving value-based care , not only stimulating physician engagement but also driving down medical cost and

PharmExec
MAY 25, 2023
The 2023 Emerging Pharma Leaders share with the Pharmaceutical Executive editors what makes a good leader, what has made them the proudest in their career, lessons they wish they had known when starting out, and more.

PharmaTech
MAY 25, 2023
Andrea Lodetti, CEO of Bormioli Pharma, an Italian pharma primary packaging manufacturer, speaks about his company's approach to the US market, supply chain instabilities, sustainability, and more.
Pharmaceutical Technology
MAY 25, 2023
Pyxis Oncology has made a definitive agreement to buy biopharmaceutical company Apexigen, in a deal valued at nearly $16m. Pyxis Oncology will issue 0.1725 shares of its common stock, with par value of $0.001 per share, for each Apexigen share. Apexigen discovers and develops a new generation of antibody therapeutics to treat cancer, along with new immuno-oncology products to harness the immune system to combat and eradicate cancer.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content