European Clinical Supply Planning: Balancing Cost, Flexibility and Time

Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.

When a CRO is bidding on a project where clinical supplies will be one of the aspects to manage on behalf of the client via a partner, leveraging the expertise of a chosen clinical supply partner can be a valuable resource in demonstrating the CRO’s understanding of and ability to deliver upon critical drug-supply related aspects of the project, and ability to hit key milestones such as FPI for their client.

The sourcing of commercial drugs for use in clinical trials either as a comparator, rescue or co-medication can be a complex process. Sponsors can face a number of challenges when trying to obtain these critical supplies for their studies, including addressing product lead times, availability, expiry limitations, safety and cost, among others. Choosing the right partner with global sourcing, regulatory, quality and clinical packaging expertise can provide the necessary guidance to help sponsors
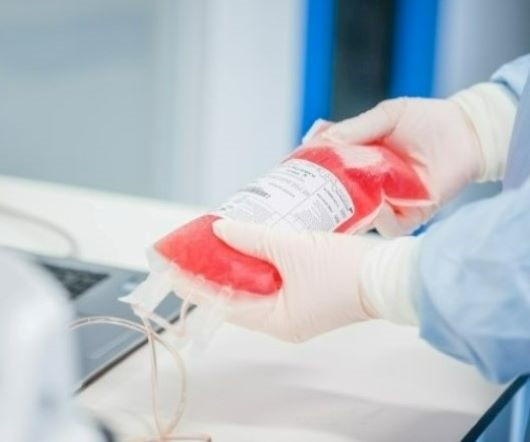
As demand for advanced therapies increases, so does the need for more specialized supply chain support, as these products have strict transportation and handling requirements. Deviations from the intended supply chain can delay timelines and affect these high value products, which can be detrimental to budgets, but more importantly can have dire effects on the patients whose lives depend on receiving these critical doses safely and on time.

Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.

ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

ZoomInfo
One of the biggest challenges for any B2B marketer is understanding your prospects’ next move — who is most likely to buy and when. Without these insights, marketing campaigns can feel more like guesswork, with high investment and little return. We’re here to tell you there’s a better way. By tracking buyers’ digital footprints and online activity, such as website visits, product reviews, and spikes in content consumption, you can engage prospects with a message that really resonates.

ZoomInfo
Consolidating your tech stack is an effective cost-saving measure that drives GTM efficiency and adds value to your enterprise. With a cohesive, integrated tech stack, your revenue teams can deliver an excellent customer experience that sets you up to win faster than your competitors.

ZoomInfo
In this eBook, we’ll run through real-world examples that show how RevOps teams can benefit from modern solutions for the access, management, and activation of their GTM data. Whether you need to improve lead response times, boost adoption of core tools, improve lead qualification, or target and automate your GTM motions, you’ll find examples of how revenue teams are solving some of the toughest problems in modern business.

ZoomInfo
Longer sales cycles. Larger buying committees. Slow-moving compliance reviews. Every go-to-market team knows the frustrations that come from a drawn-out sales process. How can you speed it up? By building a modern GTM motion that uses data, automation, and proven best practices to unlock insights, engage customers, and win faster.

ZoomInfo
Even in today’s data-driven sales world, cold calling remains a fact of life for many go-to-market professionals. Fortunately, today’s sales leaders have a crucial advantage over their predecessors: market intelligence and outreach platforms that can warm up virtually any introduction.

ZoomInfo
Longer sales cycles. Increasingly discerning buyers. More meetings. Intensifying competition. Economic uncertainty. Go-to-market teams of every size, in every industry, are grappling with these challenges firsthand. Thankfully, there’s an answer. We’ve developed an entirely new way for GTM leaders to identify and execute proven, data-driven strategies that drive revenue.

Let's personalize your content