Is the launch environment really more competitive now?
pharmaphorum
OCTOBER 7, 2022
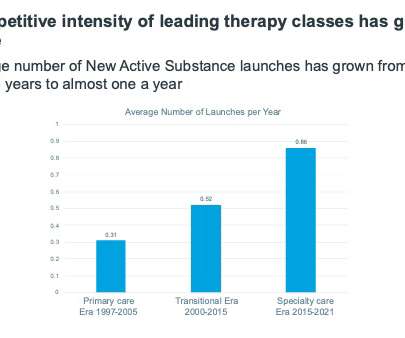
It’s often stated – mostly without reference to data – that the environment for innovative launches is more competitive now than it ever has been. A second, “transitional era” from 2000-2015 was one of older specialty products, including those in multiple sclerosis, early immunological biologics, and oncologics.






























Let's personalize your content