Moderna now in the crosshairs of Sens. Warren, Welch over price tag for COVID vaccine
Fierce Pharma
JANUARY 25, 2023
Warren, Welch over price tag for COVID vaccine kdunleavy Wed, 01/25/2023 - 13:16 Moderna now in the crosshairs of Sens.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 tag
tag  Pharma Related Topics
Pharma Related Topics 
Fierce Pharma
JANUARY 25, 2023
Warren, Welch over price tag for COVID vaccine kdunleavy Wed, 01/25/2023 - 13:16 Moderna now in the crosshairs of Sens.
Fierce Pharma
NOVEMBER 23, 2022
price tag, CSL and uniQure's hemophilia B gene therapy crosses FDA finish line. Sporting a $3.5M Wed, 11/23/2022 - 10:10.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 18, 2022
Lilly wades deeper into copycat insulin market, scoring interchangeability tag for Lantus biosim. fkansteiner. Fri, 11/18/2022 - 11:01.

World of DTC Marketing
JUNE 1, 2021
With a lot of money pouring in from MS drugs, their CEO decided that they needed to act like big pharma and moved the company to downtown Cambridge under the guise that it was necessary to get closer to biomedical R&D. If the drug is approved you can bet that it’s going to carry a huge price tag.

Fierce Pharma
APRIL 29, 2024
The price tag includes both the approved cancer drug Qinlock and another Deciphera candidate heading to the FDA’s desk in the coming months.

Fierce Pharma
MARCH 20, 2024
After scoring an FDA nod Monday for the first gene therapy to treat the rare genetic disease metachromatic leukodystrophy (MLD) in the U.S., Kyowa Kirin and its subsidiary Orchard Therapeutics have | After scoring an FDA nod Monday for the first gene therapy to treat the rare genetic disease metachromatic leukodystrophy in the U.S.,

Fierce Pharma
MAY 14, 2024
Late Tuesday, Biogen and Eisai said they kicked off a rolling FDA submission for the subcutaneous version of Leqembi—which is currently infused—after winning a fast track tag from the agency. After suffering a setback with U.S.

World of DTC Marketing
FEBRUARY 19, 2021
When will pharma stop using programmatic online ads? NewsGuard found that 67% of the COVID misinformation sites had Google advertising tags and 30% had tags from The Trade Desk. Again and again, I have seen robust fraud and online ads being placed on sites that pharma would normally block in their system.

Fierce Pharma
SEPTEMBER 22, 2023
Merck and Eisai’s Keytruda-Lenvima tag team can’t seem to catch a break. Merck and Eisai’s Keytruda-Lenvima tag team can’t seem to catch a break.

World of DTC Marketing
SEPTEMBER 7, 2022
In pharma, growth depends on new products with hefty price tags when over 80% of voters want lower costs for their prescription drugs. Biogen’s failure should be a warning to other pharma companies. It should be a warning to other pharma companies. Now Biogen is a shell of a company selling off assets to survive.

Revosuite
JUNE 18, 2019
The post Turnover in Pharma: Revealing the Price Tag appeared first on REVO. But how does that influence a company’s bottom line? What are the consequences to that “memory loss”? According to Panopto’s.

Fierce Pharma
JULY 14, 2023
The potentially curative promise of gene therapies often carries a steep price tag. | The potentially curative promise of gene therapies often carries a steep price tag. But for a pair of personalized medicine prospects in sickle cell disease (SCD), the cost could be worth it, at least as far as ICER is concerned.

pharmaphorum
OCTOBER 3, 2022
Following news of Relyvrio’s $158,000 annual price tag, both ALS advocates and outside observers have voiced outrage at the cost. The post Amylyx ALS drug draws criticism over $158,000 price tag appeared first on. ALS affects over 30,000 people in the US. It can lead to progressive paralysis and death.

Fierce Pharma
NOVEMBER 7, 2023
But on Tuesday bluebird said that Vertex’s price tag will not factor into how the Massachusetts company will price its treatment. One potential advantage for bluebird, giving the timing, could be a chance to view its rival's pricing before deciding itself.
MedCity News
APRIL 26, 2024
million price tag, the same as a CSL Behring gene therapy already available for treating the inherited bleeding disorder. The FDA has approved Beqvez, a Pfizer gene therapy developed for moderate-to-severe hemophilia B. The one-time treatment carries a $3.5

PharmExec
MARCH 29, 2024
OncLive serves as the connection to oncology, including groundbreaking cancer news and interviews with top oncologists in multimedia formats.
Pharmaceutical Technology
NOVEMBER 9, 2022
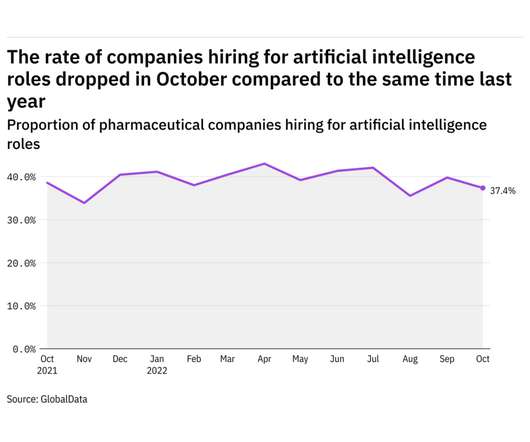
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends.

PM360
JUNE 26, 2023
Auto-tagging, a laborious task, is another area where generative AI/LLMs can assist. Auto-tagging helps the right content get delivered to the appropriate customers. Generative AI has already proven its ability to automate content tagging, which saves creators tremendous time and effort, as well as reduces the possibility of mistakes.

pharmaphorum
JULY 23, 2019
Customer journeys in pharma are changing, with important implications for the marketing supply chain. The expert panel will consider exclusive research carried out by Tag and pharmaphorum across the US, Europe and Asia-Pacific among senior decision-makers in pharma and FMCG marketing.

Contrarian Sales Techniques
OCTOBER 19, 2023
In the ever-evolving world of healthcare, the term "value" has taken on a myriad of meanings. It's not just about the price anymore. It's about the tangible and intangible benefits that medical products and services bring to the table. But who gets to define this elusive concept of "value"?

pharmaphorum
NOVEMBER 27, 2022
Supply chain challenges take many forms in pharma – from capacity issues, to managing excess safety stock, and ensuring accurate supplier communication. The post How automation can help to address supply chain challenges in pharma appeared first on. Here’s how applying automaton in pharmaceutical labs can help address these.

World of DTC Marketing
SEPTEMBER 24, 2021
Then they decided to move their HQ to an expensive campus in downtown Cambridge to play with “big pharma” Along with the move came new competitors and MS patients who reported that other MS drugs were better. They had a new MS drug that was the talk of the industry and an exciting pipeline.
Pharmaceutical Technology
JULY 8, 2022
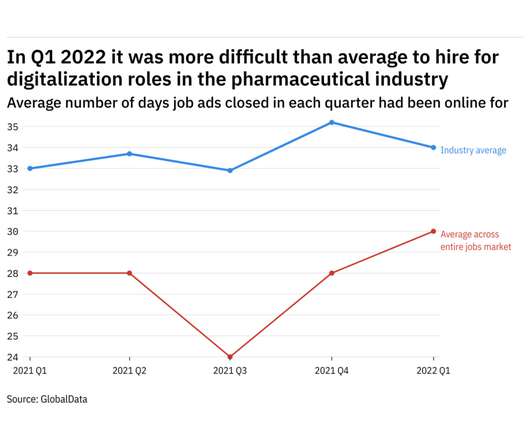
GlobalData's job analytics database tracks the daily hiring patterns of thousands of companies across the world, drawing in jobs as they're posted and tagging them with additional layers of data on everything from the seniority of each position to whether a job is linked to wider industry trends.
World of DTC Marketing
JULY 20, 2022
Pfizer, for instance, hiked the cost of its leukemia medication Besponsa again this month, bringing its per-vial price tag to $21,056. The pharma industry’s latest price hikes demonstrate why the Senate must pass the comprehensive drug pricing reforms in the reconciliation package. inflation rate.

Pharmatutor
MARCH 26, 2022
Shree Swaminarayan Sanskar Collage of Pharmacy Zundal, Gandhinagar, Gujarat, Email Id: manishakotadiya3@gmail.com, FTF Pharma Pvt. MANISHA KOTADIYA*, JAYDEEP SAVALIYA. Ahmedabad, Email ID: jaydeepsavaliya27@gmail.com. Sat, 03/26/2022 - 17:13.
pharmaphorum
JANUARY 31, 2023
Given the high price tags placed on many medications, it’s easy to understand why patients often feel that they are being taken advantage of. Pharma’s bottom line will improve. In this future, everyone wins – patients, pharma companies, physicians, and the overall healthcare ecosystem.

MedCity News
JULY 1, 2022
The $100 million price tag is in the neighborhood of the going rate for these vouchers, which grant a company a shorter regulatory review timeline for a drug that addresses a rare or neglected disease. Novartis is acquiring an FDA priority review voucher from Mallinckrodt Pharmaceuticals.

Pharmatutor
DECEMBER 27, 2021
*Shree Swaminarayan Sanskar Collage of Pharmacy Zundal, Gandhinagar, Gujarat, Email Id: manishakotadiya3@gmail.com, FTF Pharma Pvt. Ahmedabad, Email ID: jaydeepsavaliya27@gmail.com. DOWNLOAD AS PDF. Mon, 12/27/2021 - 15:34. Read more about AROMATASE INHIBITORS IN BREAST CANCER Log in or register to post comments

pharmaphorum
OCTOBER 18, 2022
Medtech giant Beckton Dickinson (BD) has signed a deal with France’s Biocorp to use the latter’s near-field communication (NFC) tags in injectable devices. The Injay tag can confirm a complete injection and transfer that information via an NFC reader to a smartphone or tablet for review by a healthcare professional.

European Pharmaceutical Review
MAY 22, 2023
Benefits of blockchain technology in the pharma supply chain Blockchain technology can address these issues in the pharmaceutical supply chain by providing timely data and increase shared data’s authenticity, integrity , and invariability. For instance, these drugs must be equipped with real-time insights.

PM360
OCTOBER 13, 2023
Another capability made possible by fine-tuning is the enhancement of content tagging. Content tagging has always been a labor-intensive task. Auto-tagging ensures that the right content efficiently reaches the right customers. Fine-tuning models can target domains and generate prompts to tag the content into relevant topics.

World of DTC Marketing
SEPTEMBER 9, 2021
If pharma is a business, they will find a way to develop, buy, or acquire new drugs. A study put a price tag on American’s bad eating habits: $50 billion a year in health care costs, attributable to cardiometabolic diseases such as heart disease, stroke, and type 2 diabetes. Then there is the cost of unhealthy Americans.

Contrarian Sales Techniques
DECEMBER 4, 2023
In the realm of pharma, where the presentation of drug samples is as critical as the information relayed, these bags serve a pivotal role. Personalize with Name Tags or Initials: A Personal Touch Adding a name tag or your initials can give your sample bag a personal touch.

World of DTC Marketing
JUNE 23, 2021
Source: Fierce Pharma If that enthusiasm pans out in the long run, it could mean at least 300,000 patients taking the drug, or $10 billion to $15 billion in peak Aduhelm from U.S. Not to mention the $56,000 annual price tag. neurologists or psychiatrists currently treating about 12,000 Alzheimer’s patients.

World of DTC Marketing
NOVEMBER 3, 2020
An #NHLBI-funded study put a price tag on American’s bad eating habits: $50 billion a year in health care costs, attributable to cardiometabolic diseases such as heart disease, stroke and type 2 diabetes. Pharma companies should be required to add content for health conditions that can be prevented by eating right and exercising.
Pharmaceutical Technology
DECEMBER 5, 2022
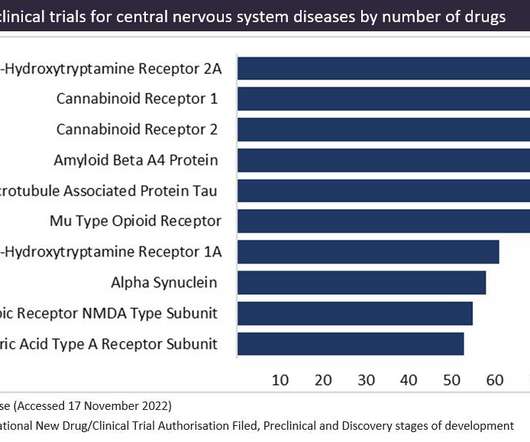
Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. This is closely followed by CB2 receptors in second place.
Pharmaceutical Technology
JULY 14, 2022
Due to the high price tags associated with these speciality medicines, innovators have naturally favoured big markets with high GDP such as the US and EU-5 (Germany, France, Spain, Italy, and the UK). Go-to-market strategies. The choice of where to launch an orphan drug is an important and difficult decision.

pharmaphorum
AUGUST 21, 2022
There’s no word yet on its pricing, but for comparison Spravato was launched with an annual price tag of around $32,400, which the Institute for Clinical and Economic Review (ICER) concluded was around 25% to 52% too high to be cost effective. Following after is AXS-12 (reboxetine) for narcolepsy, which could be filed in 2023.

Celeritas
NOVEMBER 7, 2022
In conjunction with tags, facial expression readers, or electromagnetic RFID tags, drones can monitor the health indicators of individual herd members. From satellites to mounted ear tags, sensors can be used for spectral, spatial, and radiometric applications across pastoral land. Satellite-based monitoring. Mounted Devices.
Pharmaceutical Technology
FEBRUARY 10, 2023
This is not the first treatment to come with a high price tag. On November 22, 2022, the FDA approved CSL Behring’s Hemgenix (etranacogene dezaparvovec), the first gene therapy treatment for hemophilia B, with a staggering manufacturer price of $3.5

Pharmaceutical Technology
FEBRUARY 22, 2023
The patent battle between Amgen and Sanofi over their cholesterol-lowering antibodies has divided big pharma in the past months. Both drugs come with a high price tag. This case has broader implications for pharma as an industry, which explains the interest from other businesses.
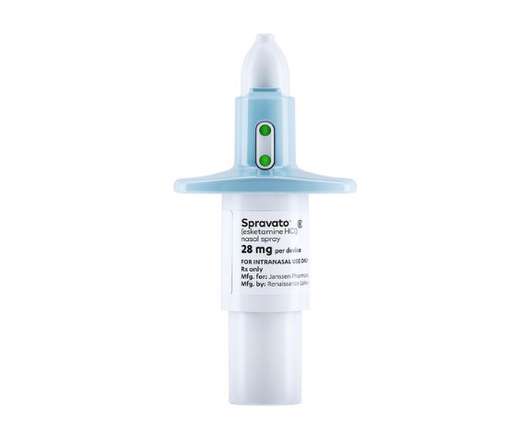
pharmaphorum
NOVEMBER 24, 2022
One issue has been the cost of the drug, with health technology assessment agency NICE in the UK and the ICER organisation in the US both concluding its price tag means it is not a cost-effective option for health systems. and 17.6%, respectively, according to J&J’s pharma unit Janssen. and 14.1%, respectively.
pharmaphorum
DECEMBER 23, 2022
price tag of $475,000 when it was first launched in 2017 – and it becomes apparent that these may not be desirable treatment options for every patient and in every setting. Add to this the considerable cost of these medications – the first approved CAR-T, Novartis’ Kymriah (tisagenlecleucel), had a U.S.
PM360
OCTOBER 28, 2022
Pharma marketers have to deal with a lot of headache-inducing complexities and restrictions that don’t impede their counterparts in less regulated industries. First, as every pharma marketer knows, Medical Legal Regulatory (MLR) review and approval is a legal layer of consumer protection, confidence, and clarity.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content