U.S. market access landscape: A push for control is erasing the boundary between payers and providers in U.S. healthcare
Clarivate
APRIL 24, 2023
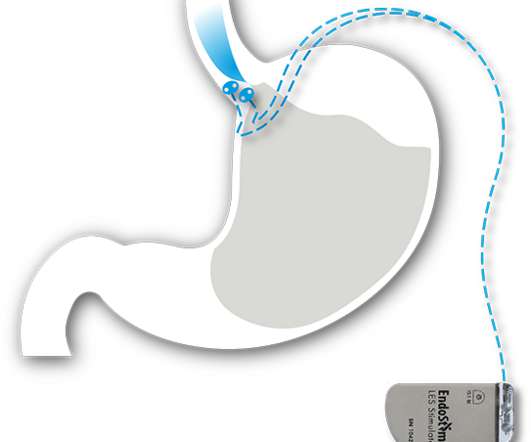
CVS then began affiliating with health systems across the country (like Cleveland Clinic ) for physician oversight of MinuteClinic’s staffed midlevel providers, just as Walgreens was rising as a formidable competitor in the convenient care space through its own walk-in clinics that it launched in fall 2006.



































Let's personalize your content