Medinol Receives FDA Approval for Next Generation EluNIR-PERL™ Drug-Eluting Coronary Stent System
Legacy MEDSearch
OCTOBER 30, 2023
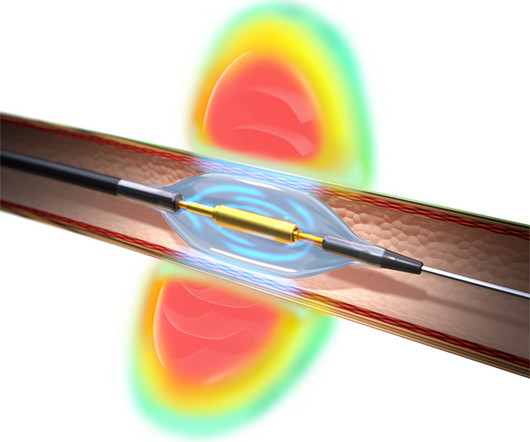
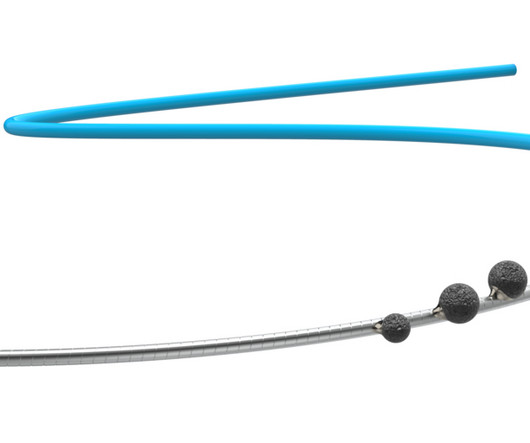
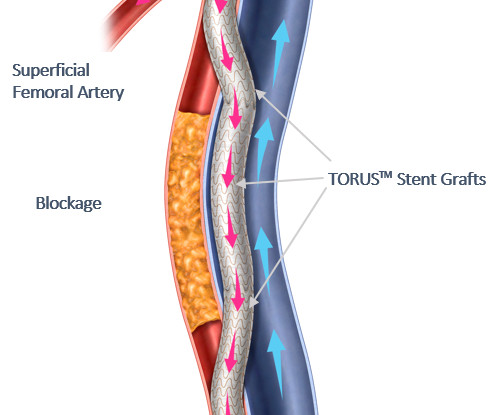
Medinol today announced United States Food and Drug Administration (FDA) approval for the EluNIR-PERL drug-eluting stent (DES) for the treatment of coronary artery disease. EluNIR-PERL builds upon the proven performance and clinical data of the EluNIR DES system. For more information, see www.medinol.com.








































Let's personalize your content