New FDA head needs to examine the accelerated approval program
World of DTC Marketing
OCTOBER 21, 2021
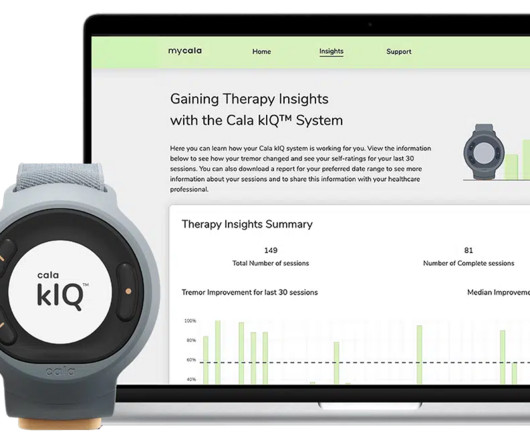
The FDA’s “accelerated approval” program expedites the evaluation process for new treatments so that patients can have access to them sooner. Between 2011 and 2018, cumulative spending on 44 FDA-approved oral targeted therapy drugs was $3.5 appeared first on World of DTC Marketing.com.











































Let's personalize your content