AstraZeneca withdraws US COVID vaccine application, shifts focus to antibody treatments
Fierce Pharma
NOVEMBER 10, 2022
AstraZeneca withdraws US COVID vaccine application, shifts focus to antibody treatments. aliu. Thu, 11/10/2022 - 09:02.

Fierce Pharma
NOVEMBER 10, 2022
AstraZeneca withdraws US COVID vaccine application, shifts focus to antibody treatments. aliu. Thu, 11/10/2022 - 09:02.
Pharmaceutical Technology
NOVEMBER 25, 2022
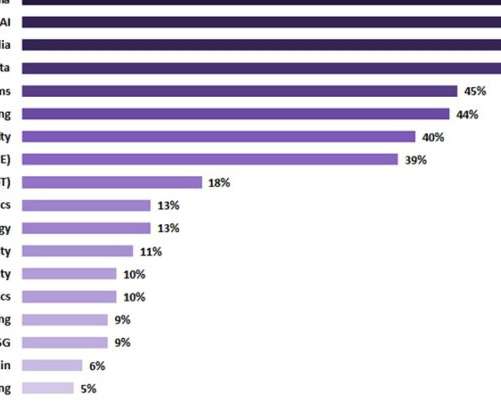
Artificial intelligence (AI) was seen as one of the top current investment priorities and was thought to continue to attract investment in the healthcare sector in the upcoming two years, according to GlobalData's latest report ‘Digital Transformation and Emerging Technology in the Healthcare Industry - 2022 Edition’. In this survey-based report tracker, digital media was prioritised as a top current investment target, with 53% of surveyed respondents confirming that their companies are currentl
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
NOVEMBER 1, 2022
Large employers, particularly self-insured companies, can demand a better and fairer system. They can use their contracting power to insist that their plans cover all FDA approved drugs.
European Pharmaceutical Review
NOVEMBER 21, 2022
The first-in-human gene therapy trial for heart failure patients with preserved ejection fraction (HFpEF) has been approved by the US Food & Drug Administration (FDA). SRD-001, the adenovirus associated virus (AAV)-based gene therapy is delivered to cardiac ventricular muscle cells via an intracoronary infusion system (produced by Sardocor, a clinical-stage gene therapy subsidiary of Medera), to increase the protein expression and functional activity of sarcoplasmic reticulum calcium ATPase

Copyright Clearance Center
NOVEMBER 7, 2022
November 7, 2022, LEHI, Utah — CloudSource+ , the newest content solution for open access materials from SirsiDynix , has integrated with Get It Now from CCC (Copyright Clearance Center) to provide immediate purchase and delivery of full-text articles to unsubscribed journals. Get It Now is used by hundreds of academic libraries around the world to help expand their virtual collections.

pharmaphorum
NOVEMBER 2, 2022
Digital therapeutics are rapidly coming into the foreground to treat a variety of conditions. Ben Hargreaves discovers how chronic pain could be a key area for digital therapeutics, as they offer non-addictive and effective relief from the condition. The struggle to manage pain for individuals has been one that goes back a long way in history, with one of the earliest recorded medical prescriptions being for opium.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
NOVEMBER 14, 2022
In a field dominated by antibodies and small molecules, two cell-therapy based approaches have come under the spotlight for showing early signs of efficacy in treating lupus. In September, a group from Friedrich Alexander University Erlangen-Nuremberg reported that five patients with lupus achieved remission after an infusion of autologous chimeric antigen receptor (CAR)-T cells led to a deep depletion of B cells.

MedCity News
NOVEMBER 17, 2022
The scheme started in New York but became a complex international endeavor as the two men attempted to launder money received from fraudulent Medicare claims for cancer drugs during the pandemic.

Medgadget
NOVEMBER 11, 2022
Researchers at the University of Texas at Arlington, in collaboration with Shani Biotechnologies, a local firm, have created a point-of-care device that can accurately measure hemoglobin levels and perform pulse oximetry in individuals with dark skin. At present, methods to determine hemoglobin levels at the point of care, such as pulse oximetry, are inaccurate in individuals with higher levels of melanin in their skin, and there is a clear need to develop alternatives that work for everyone.
European Pharmaceutical Review
NOVEMBER 2, 2022
Researchers at Great Ormond Street Hospital for Children (GOSH) and University College London (UCL) Great Ormond Street Institute of Child Health (UCL GOS ICH) have used “universal” CRISPR-edited cells in humans for the first time to treat B-cell acute lymphoblastic leukaemia (B-ALL) child patients with engineered donor T cells. The treatment did not require matching donor cells, a major step in developing gene-edited cells for cancer treatment.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
pharmaphorum
NOVEMBER 15, 2022
A new survey conducted by juli, an AI-powered chronic condition platform, has revealed that the value placed on digital privacy differs widely among US users. With the HLTH 2022 event now well underway, digital health industry leaders all gathered together to attend in Las Vegas this week, the juli survey results reveal consumer responses to be more varied than one would have expected when it comes to data privacy.
Fierce Pharma
NOVEMBER 8, 2022
Moderna’s Spikevax carries higher risk of myocarditis than Pfizer's Comirnaty, study says. zbecker. Tue, 11/08/2022 - 11:03.

PharmExec
NOVEMBER 8, 2022
Why enabling a shared culture of quality across an organization is key to maintaining pharma’s recent pace of innovation wins—while inspiring new levels of confidence.

MedCity News
NOVEMBER 9, 2022
More than 31% of people with employer-sponsored insurance stuck with a job they disliked for the company’s health insurance, a Forbes Advisor survey found. Another 8% of respondents left a job they liked to seek better coverage.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

PharmaVoice
NOVEMBER 11, 2022
Recorded just weeks before his passing, our sit-down interview with the legendary medical sciences entrepreneur showcased his passion for pushing the industry toward its next frontier.
European Pharmaceutical Review
NOVEMBER 23, 2022
The US Food and Drug Administration (FDA) has approved Hemgenix (etranacogene dezaparvovec), the first gene therapy for adults with Haemophilia B (congenital Factor IX deficiency) who currently use Factor IX prophylaxis therapy, or have current or historical life-threatening haemorrhage, or have repeated, serious spontaneous bleeding episodes. Standard treatment for the condition involves replacing the missing or deficient clotting factor to improve the body’s ability to stop bleeding and promot
pharmaphorum
NOVEMBER 7, 2022
Blood cells grown in a laboratory have been given to people for the first time in a clinical trial being carried out by researchers in the UK, in the hope that plentiful supplies of rare blood groups can be manufactured to order. A team from the universities of Bristol and Cambridge, NHS trusts and NHS Blood and Transplant (NHSBT) have started giving small quantities of the lab-grown red blood cells – a couple of teaspoons full – to two healthy volunteers to see if they are safe.
Fierce Pharma
NOVEMBER 23, 2022
J&J's nasal spray Spravato bests former AZ drug Seroquel XR in treatment-resistant depression trial. fkansteiner. Wed, 11/23/2022 - 09:26.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Pharmaceutical Technology
NOVEMBER 29, 2022
The National Health Service (NHS) in England, UK, has expedited the rollout of Bayer ’s new life-extending drug, darolutamide, to treat the most advanced kinds of prostate cancer that have spread to other body parts. With the latest development, NHS will become the first healthcare system in Europe to offer this drug to prostate cancer patients. Nearly 9,000 men with prostate cancer will be eligible to receive this treatment.

MedCity News
NOVEMBER 10, 2022
Sprinter Health is integrating its at-home clinical and diagnostic services into Firefly Health’s virtual primary care model. Firefly’s members will be able to receive common medical services from Sprinter clinicians in their homes — such as vital checks, blood draws, electrocardiograms, diabetic eye exams and diabetic foot screenings.
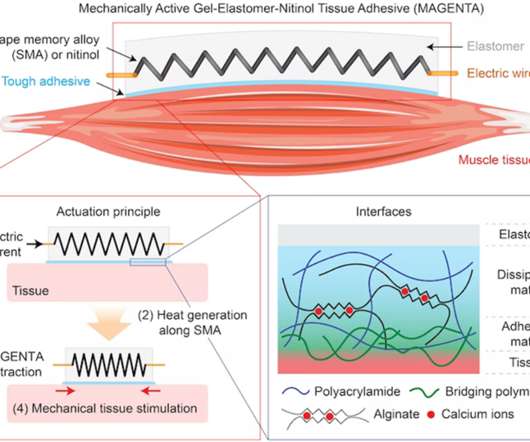
Medgadget
NOVEMBER 16, 2022
Scientists at the Wyss Institute for Biologically Inspired Engineering at Harvard have developed a mechanically active gel-elastomer-nitinol tissue adhesive, otherwise known as MAGENTA. The implantable device functions as a soft robot, and it can be adhered to the outside of a muscle. When an electrical charge is applied to the device, a spring inside made from nitinol (a shape memory alloy) heats up and begins to actuate, creating a contraction and stretching effect on the attached muscle.

European Pharmaceutical Review
NOVEMBER 18, 2022
Upstaza (eladocagene exuparvovec) is the first and only approved treatment for aromatic L-amino acid decarboxylase (AADC) deficiency and is the first marketed gene therapy for direct infusion into the brain. The product, produced by biopharma company PTC Therapeutics, is approved for patients 18 months and over. It has been granted marketing authorisation by the Medicines and Healthcare Products Regulatory Agency (MHRA) in Great Britain.

pharmaphorum
NOVEMBER 16, 2022
Novartis has already spun out its eyecare business Alcon, and is now considering the sale of its ophthalmology and respiratory medicines businesses as it continues a narrowing of its focus, according to media reports. A Bloomberg article citing people close to the matter claims that Novartis is engaged in early discussions about the sale of the two units, which could raise billions of dollars in capital that could be reinvested into the pharma group’s pipeline.
Fierce Pharma
NOVEMBER 23, 2022
Sporting a $3.5M price tag, CSL and uniQure's hemophilia B gene therapy crosses FDA finish line. zbecker. Wed, 11/23/2022 - 10:10.
Pharmaceutical Technology
NOVEMBER 2, 2022
Pfizer has reported a 6% decline in revenue to $22.6bn in the third quarter (Q3) of 2022 as against $24bn in the same quarter last year. In the quarter, revenues rose 2% operationally on omitting contributions from Covid-19 therapies, Paxlovid and Comirnaty. Reduction in revenues from Comirnaty outside the US and reduced revenues for some Comirnaty-linked manufacturing works carried out on behalf of BioNTech, Xeljanz and Sutent worldwide were the key drivers that contributed to the revenue dec

MedCity News
NOVEMBER 22, 2022
Based on some independent research that my company commissioned with more than 200 companies, we came to several conclusions that are highlighted in the article. Broadly, however, it signals the false sense of (cyber)security that many companies are currently harboring.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

PharmaVoice
NOVEMBER 28, 2022
After nearly five years, the homicide case involving pharma billionaires continues to perplex investigators.

European Pharmaceutical Review
NOVEMBER 16, 2022
As the emphasis of new drug development gravitates towards new, ground-breaking therapies and vaccines, the requirements of manufacturing sites, equipment and processes need to be appropriate for this new environment. For young biotechs, scaling up production brings green-field challenges, while for more established pharma a shift may be required to ensure that facilities are optimised for new ways of working.
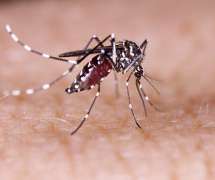
pharmaphorum
NOVEMBER 23, 2022
The US regulator has started a priority, six-month review of Takeda’s dengue fever vaccine TAK-003 – tipped as a potential blockbuster product – making a decision likely in the first half of 2023. The Japanese pharma said it has filed for approval of TAK-003 with the FDA for the prevention of dengue disease caused by any dengue virus serotype in individuals aged four through 60.

Fierce Pharma
NOVEMBER 7, 2022
GSK's CAR-T rival Blenrep fails multiple myeloma trial, endangering its accelerated approval. aliu. Mon, 11/07/2022 - 09:45.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content