GSK hepatitis B hope bepirovirsen heads for phase 3 test
pharmaphorum
JUNE 27, 2022
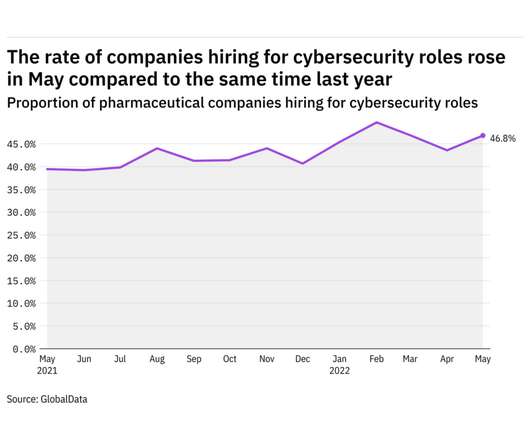
An antisense drug in development at GSK has shown further activity against hepatitis B virus (HBV) in a mid-stage trial, setting up a phase 3 assessment next year to see if it could offer a “functional cure” for the widespread disease. Interim results from the phase 2b B-Clear showed that bepirovirsen reduced levels of hepatitis B surface antigen (HBsAg) and HBV DNA after 24 weeks’ treatment to below the lower limit of detection in people with chronic hepatitis B, raising hopes
































Let's personalize your content