Do patients care about accelerated approval drugs?
World of DTC Marketing
MARCH 23, 2022
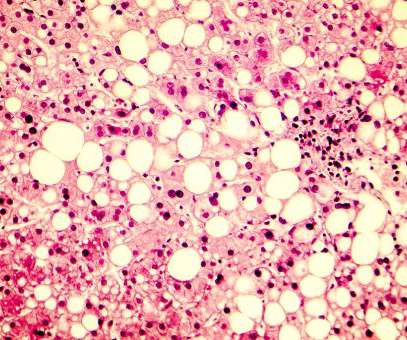
Do patients care? Accelerated Approval was developed in 1992 in response to the HIV/AIDS crisis and has led to expedited drug and biologic approvals in several disease areas across the FDA. Do patients care? Should patients be made aware that the drug they are being prescribed is an “accelerated approval drug”?










































Let's personalize your content