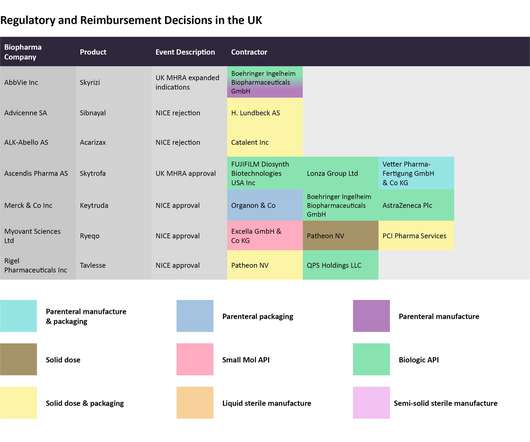
Innovation key for boosting UK biopharma competitiveness
European Pharmaceutical Review
JULY 7, 2023
New recommendations to boost the competitiveness in UK biopharma and Medtech sectors have been published by Imperial College London. Technological solutions and policy recommendations for government have been identified as a way to help make sectors such as biopharma and Medtech more innovative and increase their value added.












































Let's personalize your content