FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy
Fierce Pharma
MAY 31, 2023
FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy kdunleavy Wed, 05/31/2023 - 13:02
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
Fierce Pharma
MAY 31, 2023
FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy kdunleavy Wed, 05/31/2023 - 13:02

Fierce Pharma
APRIL 26, 2023
FDA argues AstraZeneca, Merck's Lynparza only works in subset of prostate cancer patients aliu Wed, 04/26/2023 - 14:19
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
DECEMBER 20, 2023
FDA OKs test to spot patients at risk of opioid use disorder Phil.Taylor Wed, 20/12/2023 - 10:14 Bookmark this

Pharmatutor
JANUARY 12, 2024
Overview of FDA Drug approvals in 2023 - Total Guide admin Sat, 01/13/2024 - 11:38 In 2023, FDA approved 55 new drugs never before approved or marketed in the U.S., We also made other important approval decisions, such as expanding the use or patient population of previously approved drugs. known as “novel” drugs.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
A recent draft from the FDA provides valuable insight. Learning Objectives: Dose-response curves and patient treatment: How do pharmacologic and toxicologic principles apply to the dosing of drugs in clinical development? What will the future hold for clinical research?

European Pharmaceutical Review
JANUARY 29, 2024
N-Nitrosamines By far the biggest issue bedevilling industry during 2023 was the continuing N-nitrosamine contamination saga, which was covered in the third issue of EPR 2023. 4 The use of CPCA assessments allows for the assignment of scientifically justifiable higher acceptable intakes (AIs), without impacting patient safety.

pharmaphorum
AUGUST 17, 2022
The clock is now ticking on the FDA’s review of GSK’s momelotinib for myelofibrosis patients with anaemia – the centrepiece of its $1.9 The US regulator is due to make a decision on momelotinib by 16 June, 2023, on the basis of phase 3 results reported in January, said GSK.

PharmaTech
SEPTEMBER 19, 2023
Maik Jornitz, Principal Consultant, BioProcess Resources LLC, discusses the definition of patient safety and how to implement new technologies into upgraded facilities.

Fierce Pharma
JUNE 28, 2023
With a spring approval in hand, Reata Pharmaceuticals has been waiting patiently to launch its first commercial product, Skyclarys. After an FDA thumbs up on Reata's approval supplement for Skyclarys, analysts feel confident the newly commercial-stage company can meet—or potentially even exceed—a $45.9
European Pharmaceutical Review
OCTOBER 18, 2023
The US Food and Drug Administration (FDA) has approved XPHOZAH ® (tenapanor), the first and only phosphate absorption inhibitor. Overall, the data showed that XPHOZAH significantly reduced elevated serum phosphorus in patients receiving maintenance hemodialysis.

pharmaphorum
DECEMBER 22, 2022
The life sciences industry is flexing towards innovation in new areas, faster than ever before, and increasing patient care in astonishing ways. We can now measure patient activity, steps, and movement continuously and in real time, which serves as a new potential indicator of treatment effectiveness.

European Pharmaceutical Review
JANUARY 10, 2023
Clarivate Plc has released its Drugs to Watch 2023 report — among 70 of the drugs highlighted, including potential blockbuster drugs, the majority were revealed to be personalised medicines. The report offers predictive analysis of drugs entering the market or launching key indications in 2023.
Pharmaceutical Technology
JUNE 13, 2023
The US Food and Drug Administration (FDA) has accepted AstraZeneca’s new drug application (NDA) for the combination of capivasertib and FASLODEX (fulvestrant), and granted it priority review. The regulator will announce its decision during the fourth quarter of 2023.
Pharmaceutical Technology
SEPTEMBER 21, 2023
On 15 September 2023, the US Food and Drug Administration (FDA) approved GlaxoSmithKline’s (GSK’s) Janus kinase (JAK) inhibitor, Ojjaara (momelotinib), for the treatment of intermediate or high-risk myelofibrosis in adults with anaemia.

Pharmaceutical Technology
MARCH 2, 2023
The US Food and Drug Administration (FDA) has approved Reata Pharmaceuticals ’ oral, once-daily medication SKYCLARYS (omaveloxolone) to treat Friedreich’s ataxia patients. There are three more drug candidates with major trial readouts that are expected in 2023.
European Pharmaceutical Review
NOVEMBER 6, 2023
IZERVAY ( avacincaptad pegol intravitreal solution) induced a year-over-year reductions in the rate of geographic atrophy lesion growth in patients with geographic atrophy secondary to age-related macular degeneration (AMD) in a Phase III trial, Astellas Pharma has announced. Astellas to accelerate ocular disease treatments with $5.9

Pharmaceutical Technology
APRIL 17, 2023
On 14 April 2023, experts from the US Food and Drug Administration’s (FDA) Advisory Committee (AdCom) voted largely in favour of the potential approval of Otsuka’ s and Lundbeck Pharmaceuticals’ Rexulti for the treatment of agitation associated with Alzheimer’s dementia (AAD). Rexulti is an atypical antipsychotic.

PM360
OCTOBER 13, 2023
HCPs need pharma, and pharma needs HCPs—so let’s make sure that this next chapter of engagement is one of partnership, in pursuit of the interest of patients.” Only 15% of prescribers think the drugs come at reasonable cost to patients or have decent insurance coverage eligibility. Three quarters of U.S.

European Pharmaceutical Review
JANUARY 8, 2024
Commercial manufacturing of Novartis’ radioligand therapy Pluvicto TM (INN: lutetium ( 177 Lu) vipivotide tetraxetan / USAN: lutetium Lu 177 vipivotide tetraxetan) at its largest and most advanced manufacturing facility for these treatments manufacturing in the world, has been approved the US Food and Drug Administration (FDA).
Pharmaceutical Technology
JUNE 9, 2023
Biopharmaceutical company Novaliq has received approval from the US Food and Drug Administration (FDA) for VEVYE (cyclosporine ophthalmic solution) 0.1% It has been designed to address the unmet needs of patients and to provide quick action and well-tolerated dry eye drug therapy. to treat the signs and symptoms of dry eye disease.
Pharmaceutical Technology
MAY 26, 2023
The US Food and Drug Administration (FDA) has granted priority review for Takeda and HUTCHMED’s new drug application (NDA) for fruquintinib. This 691-patient study was conducted in Europe, the US, Japan and Australia. The FDA has now assigned a prescription drug user fee act action date of 23 November 2023 for the NDA.
pharmaphorum
DECEMBER 19, 2022
The US Food and Drug Administration (FDA) has approved Swiss drugmaker Ferring Pharmaceuticals’ Adstiladrin (nadofaragene firedenovec-vncg) for the treatment of adult patients with high-risk Bacillus Calmette-Guérin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumours.
Pharmaceutical Technology
MAY 29, 2023
Lexicon Pharmaceuticals (Lexicon) has received approval from the US Food and Drug Administration (FDA) for its Inpefa drug to treat heart failure. The SOLOIST-WHF (worsening heart failure) and SCORED trials together assessed around 12,000 patients.

European Pharmaceutical Review
JANUARY 20, 2023
The US Food and Drug Administration (FDA) has issued a complete response letter for the accelerated approval submission of donanemab for early Alzheimer’s, Eli Lilly and Company revealed. Lilly confirmed that the Phase III trial included more than 100 patients treated with donanemab.

Pharmaceutical Technology
JUNE 19, 2023
UK-based pharmaceutical giant GSK has announced that the US Food and Drug Administration (FDA) has extended the review period of its new drug application (NDA) for the rare bone cancer drug momelotinib by three months. The FDA’s review will take into account data from the Phase III MOMENTUM trial (NCT04173494).

European Pharmaceutical Review
JANUARY 6, 2023
The US Food and Drug Administration (FDA) has granted priority review for glofitamab, Roche’s CD20xCD3 T-cell engaging bispecific antibody. The decision for the FDA priority review of glofitamab is for adults with relapsed or refractory (R/R) large B-cell lymphoma (LBCL) after two or more lines of systemic therapy.

PM360
DECEMBER 14, 2023
A standard dose of the medicine causes acute kidney damage in 20% of patients and 50% of those who receive higher dosages used to treat life-threatening infections on a broad spectrum. for adult patients with progressing desmoid tumors who require systemic treatment. The tablets are created by SpringWorks Therapeutics Inc.

PharmaTimes
OCTOBER 31, 2022
A final decision for daprodustat by the FDA is expected by 1 February 2023

pharmaphorum
JANUARY 27, 2023
AstraZeneca’s revenue boost from COVID-19 therapy Evusheld looks set to be curbed early, as the FDA withdraws authorisation for the antibody on the grounds that it is ineffective against most subvariants now circulating in the US. and other XBB subvariants, said the FDA in an update. Evusheld is also ineffective against the BQ.1,
Pharmaceutical Technology
MAY 10, 2023
Synlogic has received orphan drug designation (ODD) from the US Food and Drug Administration (FDA) for SYNB1934 to treat phenylketonuria (PKU), a rare inherited metabolic disease. The orally administered, non-systemically absorbed drug candidate SYNB1934 has been designed for reducing blood phenylalanine (Phe) levels in PKU patients.
Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has granted rare paediatric drug designations for IPS HEART’s stem cell therapeutics, GIVI-MPC and ISX9-CPC, to treat Duchenne muscular dystrophy (DMD) patients. The drug candidate received an orphan drug designation (ODD) from the FDA in February 2023.

PM360
SEPTEMBER 7, 2023
HCPs are depending on developers’ promised changes to make the platform more patient focused. Also, the platform holds promise as a more effective way for healthcare professionals to engage directly with patients. Despite these concerns, some HCPs are feeling positively about Threads. Ask me anything!” Tarsus Pharmaceuticals, Inc.
Pharmaceutical Technology
JUNE 1, 2023
The US Food and Drug Administration (FDA) has granted a combination of AstraZeneca and MSD ’s Lynparza (olaparib), with standard therapies for treating BRCA-mutated (BRCAm) metastatic castration-resistant prostate cancer (mCRPC). So far, 68 trials have been initiated in 2023.
Pharmaceutical Technology
JUNE 21, 2023
The US Food and Drug Administration (FDA) has granted Teikoku Pharma’s dexmedetomidine transdermal system a fast track designation. With an estimated enrollment of 182 patients undergoing abdominoplasty, the company will present data comparing its transdermal system with a placebo in Q3 of 2023.
European Pharmaceutical Review
AUGUST 14, 2023
The US Food and Drug Administration (FDA) has approved Janssen’s Akeega (niraparib and abiraterone acetate), for the treatment of adult patients BRCA-positive metastatic castration-resistant prostate cancer (mCRPC). The FDA approval was based on positive results from the multi-centre Phase III MAGNITUDE study.

European Pharmaceutical Review
JUNE 11, 2023
The US Food and Drug Administration (FDA) has approved commercial production at Bristol Myers Squibb’s newest cell therapy manufacturing facility in Devens, Massachusetts. Each batch of engineered T cells is manufactured individually and infused back to the original cancer patient.
Pharmaceutical Technology
MAY 18, 2023
The US Food and Drug Administration (FDA) has accepted Ardelyx’s resubmission of a new drug application (NDA) for XPHOZAH (tenapanor) to control serum phosphate in adults with chronic kidney disease on dialysis who have had insufficient response or intolerance to a phosphate binder treatment.
Pharmaceutical Technology
FEBRUARY 10, 2023
4D Molecular Therapeutics (4DMT), the California-based biotechnology company focused on developing gene therapies for rare and large market diseases, has had the FDA place a clinical hold onto its Fabry disease (FD) gene therapy program (4D-310).
PM360
APRIL 7, 2023
Patient Pages: How Healthcare Costs Affect Insured Patients Today A new study shows an insured American with an employer-sponsored health insurance plan can expect to spend more than $320,000 (including insurance premiums and out-of-pocket costs) during his/her adult lifetime.

Pharmaceutical Technology
JUNE 21, 2023
AGEPHA Pharma has received approval from the US Food and Drug Administration (FDA) for LODOCO (colchicine, 0.5 The regulatory approval was based on the findings obtained from a double-blind, multinational, placebo-controlled, randomised clinical trial conducted in 5,522 chronic coronary disease patients.

Pharmaceutical Technology
JUNE 8, 2023
The use of 4D Molecular Therapeutics’ (4DMT) aerosolised gene therapy 4D-710 has improved the quality-of-life and spirometry-measured outcomes in three cystic fibrosis patients , based on early results from a Phase I/II study presented at this year’s annual meeting of the European Cystic Fibrosis Society (ECFS).
Pharmaceutical Technology
APRIL 4, 2023
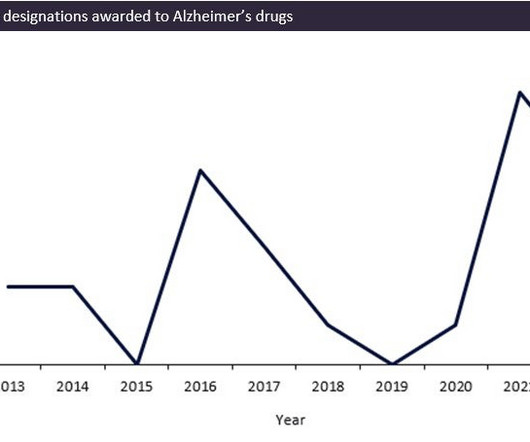
The FDA has seen a record surge in review designations being awarded over the last two years for Alzheimer’s indications, with 12 review designations being awarded to drugs between 2020 and 2022. Source: GlobalData, Drugs Database (Accessed 09 March 2023). Key successes of the FDA’s efforts include the quick development of Leqembi.

pharmaphorum
OCTOBER 27, 2022
Santhera has completed a rolling application for its Duchenne muscular dystrophy (DMD) therapy vamorolone in the US, setting up a possible approval and launch in the latter half of 2023. Its lead drug has already claimed fast track and rare paediatric disease designations from the FDA.
Pharmaceutical Technology
MAY 2, 2023
The US Food and Drug Administration (FDA) has accepted the supplemental biologics licence application submitted by Bristol Myers Squibb for Reblozyl (luspatercept-aamt) as a first-line treatment of anaemia in adults with lower-risk myelodysplastic syndromes (MDS).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content