Pharmaceutical industry: 2023 in retrospect
European Pharmaceutical Review
JANUARY 29, 2024
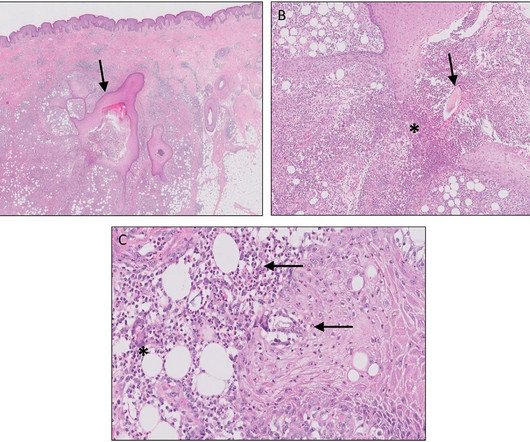
N-Nitrosamines By far the biggest issue bedevilling industry during 2023 was the continuing N-nitrosamine contamination saga, which was covered in the third issue of EPR 2023. 4 The use of CPCA assessments allows for the assignment of scientifically justifiable higher acceptable intakes (AIs), without impacting patient safety.







































Let's personalize your content