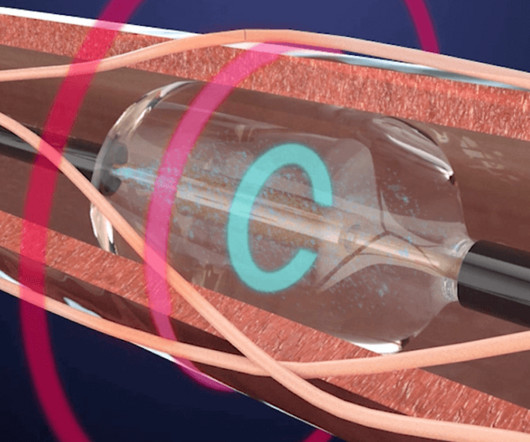
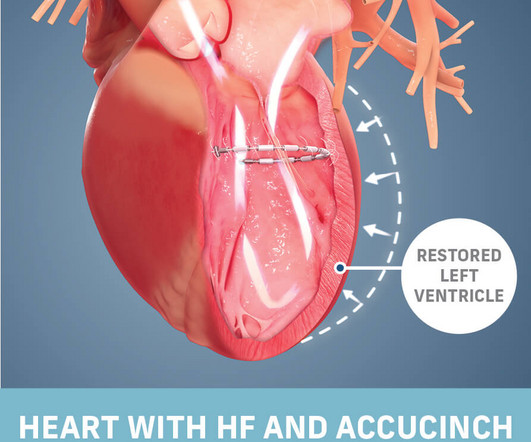
ReCor Medical Announces Two Concurrent JAMA Network Publications of Study Results on the Paradise Ultrasound Renal Denervation System for the Treatment of Hypertension
Legacy MEDSearch
FEBRUARY 28, 2023
RADIANCE II is a randomized, sham-controlled US FDA IDE pivotal trial of the Paradise uRDN System in the treatment of patients with uncontrolled hypertension. Patients were to remain off antihypertensive medications throughout the two months of follow-up unless specified BP criteria were exceeded. mmHg (p<0.0001).






































Let's personalize your content