FDA grants rare paediatric drug status for IPS HEART’s stem cell drugs
Pharmaceutical Technology
APRIL 14, 2023
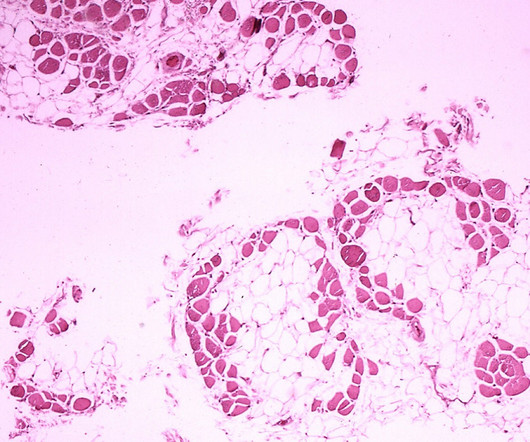
The US Food and Drug Administration (FDA) has granted rare paediatric drug designations for IPS HEART’s stem cell therapeutics, GIVI-MPC and ISX9-CPC, to treat Duchenne muscular dystrophy (DMD) patients. The drug candidate received an orphan drug designation (ODD) from the FDA in February 2023.


























Let's personalize your content