Lung cancer deaths decline but.
World of DTC Marketing
FEBRUARY 17, 2022
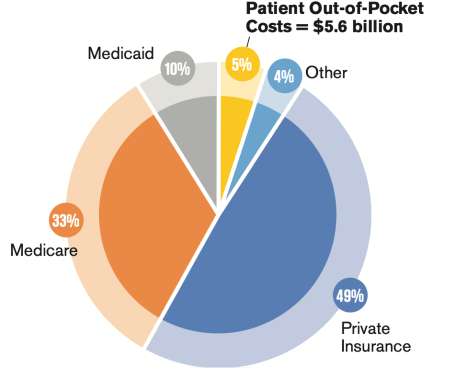
In recent years, early detection and treatment improvements have helped boost the 3-year survival rate for lung cancer from 21% in 2004 to 31% in 2015 through 2017. But it’s still the leading cause of cancer deaths. Although lung cancer’s mortality rate has fallen, the disease is still the leading cause of cancer deaths.


























Let's personalize your content