Number of ongoing US drug shortages reaches new high, pharmacist group says
Fierce Pharma
APRIL 11, 2024
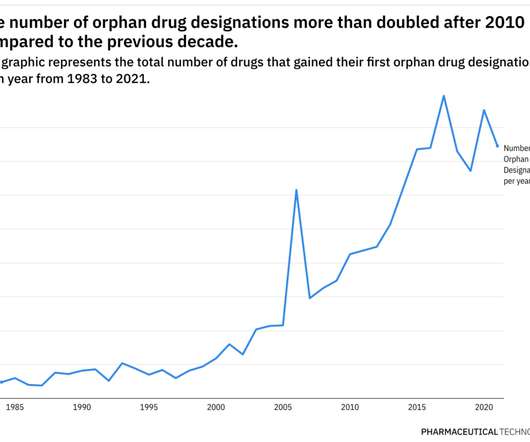
as of 2024’s first quarter—the highest number recorded since ASHP began tracking shortage data back in 2001. . | The American Society of Health-System Pharmacists has released new data showing there were 323 drugs in shortage in the U.S.







































Let's personalize your content