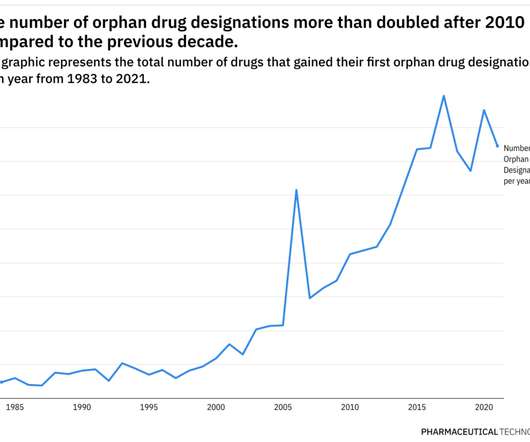
Rare Disease Spotlight – tracing the rise of orphan drug designations over almost 40 years
Pharmaceutical Technology
JUNE 29, 2022
The US Food and Drug Administration (FDA) put a high-profile bluebird bio trial for sickle cell disease on partial clinical hold, and advisory panels deliberated over decisions involving gene therapies for amyotrophic lateral sclerosis (ALS), cerebral adrenoleukodystrophy (CALD), and beta-thalassemia.












Let's personalize your content