Cold Chain: a Hot Topic for mRNA Vaccines
Pharmaceutical Commerce
AUGUST 10, 2022
With the use of mRNA vaccines on the rise, so is the need for dedicated freezing and storing solutions.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 topic vaccines
topic vaccines 
Pharmaceutical Commerce
AUGUST 10, 2022
With the use of mRNA vaccines on the rise, so is the need for dedicated freezing and storing solutions.
Pharmaceutical Technology
AUGUST 1, 2022
Moderna has entered a new supply contract with the US Government to deliver 66 million doses of its Covid-19 vaccine booster candidate, mRNA-1273.222. The contract comprises a $1.74bn award to produce and supply these vaccine doses and options to further procure up to 234 million additional doses of the company’s booster candidates.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Technology
DECEMBER 21, 2022
This Moderna Innovation and Technology Centre (MITC) is expected to offer access to a locally produced future mRNA vaccine portfolio against respiratory viruses, subject to regulatory evaluation and licensure. Additionally, the centre is anticipated to have to capacity to make up to 250 million doses of vaccines per year.

Pharma Leaders
APRIL 20, 2023
The FDA has updated emergency use authorizations for the Pfizer-BioNTech and Moderna COVID-19 bivalent mRNA vaccines to allow individuals 65 years and older to receive a second booster dose at least four months after the first bivalent dose. The FDA also authorized use of the current bivalent vaccines for everyone six months of age and older.

Pharmaceutical Technology
AUGUST 1, 2022
GreenLight Biosciences has entered a partnership with the US National Institutes of Health (NIH) for the development of Covid-19 vaccines, which offer broader protection against new variants and with durable effects. They intend to develop vaccines that provide lasting immune responses compared to existing vaccines.
Pharmaceutical Technology
JULY 15, 2022
Health Canada has granted approval for the usage of Moderna’s messenger RNA (mRNA) Covid-19 vaccine, Spikevax, in a 25µg two-dose regimen for active immunisation to prevent Covid-19 in children aged six months to five years. So far, children aged below five years were not eligible to receive the Covid-19 vaccine in Canada.
European Pharmaceutical Review
AUGUST 3, 2022
Commission has established the mRNAVAC Working Party to begin working on quality standards to support the emerging field of messenger RNA (mRNA) vaccines. The newly created Working Party’s first task will be to develop a consolidated strategy for future standards addressing these vaccines and their components.

Pharmaceutical Technology
AUGUST 2, 2022
Samsung Biologics and GreenLight Biosciences have completed the initial commercial-scale engineering run for their messenger ribonucleic acid (mRNA) Covid-19 vaccine under their manufacturing collaboration. Following the demonstration at Samsung, the clinical trial of GreenLight’s Covid-19 booster vaccine is anticipated to commence this year.

Pharmaceutical Technology
DECEMBER 18, 2022
The 0.25mL dose of the booster vaccine could potentially be used in the European Union (EU) following authorisation in these children a minimum of three months following a previous Covid-19 vaccination. 1273.214, a bivalent Omicron-targeting Covid-19 vaccine of the company. 5), mRNA-1273. Free Whitepaper.
Pharmaceutical Technology
AUGUST 15, 2022
The UK Medicines and Healthcare products Regulatory Agency (MHRA) has granted conditional authorisation for Moderna ’s Covid-19 booster vaccine, mRNA-1273.214 (Spikevax Bivalent Original/Omicron), for use in adults aged 18 years and above. It comprises mRNA-1273 (Spikevax) and a vaccine candidate that acts on the SARS-CoV-2 virus’ BA.1

European Pharmaceutical Review
AUGUST 22, 2023
The investment aims to improve vaccines and shorten the time it takes to produce medicines. Winners of a series of competitions centering on the aforementioned topics will obtain funding for UK medicines manufacturing. Seventeen new projects from the Innovate UK Transforming Medicine Manufacturing programme will benefit.

Pharmaceutical Technology
MARCH 20, 2023
RVAC Medicines has announced a research collaboration with the University of Pennsylvania (Penn) for the discovery and development of mRNA vaccines. The mRNA vaccine candidates will help reduce the chances of autoimmune responses that might lead to allergic conditions or serious autoimmune diseases.
Pharmaceutical Technology
AUGUST 28, 2022
Pfizer and BioNTech’s Covid-19 vaccine, Comirnaty, is said to have infringed patents filed by Moderna between 2010 and 2016 for its mRNA technology. This technology was vital for developing Spikevax, an mRNA Covid-19 vaccine of Moderna. 5 Omicron-targeting bivalent booster vaccine for Covid-19, mRNA-1273.222.
Pharmaceutical Technology
JUNE 21, 2023
The Drug Controller General of India (DCGI) has granted emergency use authorisation (EUA) for Gennova Biopharmaceuticals’ Omicron-specific mRNA-based Covid-19 booster vaccine, GEMCOVAC-OM. The vaccine produced significantly higher immune responses when given as a booster. GEMCOVAC-OM was developed using indigenous platform technology.

pharmaphorum
AUGUST 25, 2022
Today on the pharmaphorum podcast, Editor in Chief Jonah Comstock welcomes Hedi Ben Brahim and Eric Quéméneur, CEO and chief science officer respectively of Transgene, to discuss cancer vaccines in general and Transgene’s recent work in the space in particular. . Podbean. .

Pharmaceutical Technology
JULY 6, 2022
The mRNA technology was used for the development of the Covid-19 vaccine. CureVac sought fair compensation for infringement of a portfolio of its intellectual property rights used to produce and sell BioNTech and Pfizer’s mRNA Covid-19 vaccine, Comirnaty. Topic sponsors are not involved in the creation of editorial content.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. Topic sponsors are not involved in the creation of editorial content.

Pharma Leaders
JUNE 9, 2023
Biotech firm Promosome is the latest company to sue big pharma for infringing on its patent for messenger RNA (mRNA) technology during the rush to develop a COVID-19 vaccine, claiming in separate filings that Moderna and Pfizer reaped enormous profits using Promosome research. Promosome filed the suits Tuesday in the U.S.
Pharmaceutical Technology
OCTOBER 10, 2022
Health Canada has approved Pfizer ’s subsidiary Pfizer Canada and BioNTech’s bivalent Covid-19 vaccine, Comirnaty, as a 30?g 1-adapted bivalent vaccine. 5-adapted bivalent vaccine. 5-adapted bivalent vaccine. A clinical trial of the vaccine is currently underway in individuals aged 12 years and above.

Pharmaceutical Technology
JANUARY 20, 2023
In 2020, Moderna made a net loss of $747 million while its investigational mRNA vaccines were under development. However, during the Covid-19 pandemic, the US-based biotech rose to prominence as it was one of the first companies to develop Covid-19 vaccines with its mRNA technology. billion in Covid-19 vaccine sales.
Pharmaceutical Technology
APRIL 4, 2023
Gennova Biopharmaceuticals has applied for an emergency use authorisation (EUA) from the Drug Controller General of India (DCGI) for its mRNA-based Omicron-specific Covid-19 booster vaccine. Dubbed GEMCOVAC-OM, the needle-free vaccine will be delivered intradermally with the PharmaJet Tropis Precision Delivery System (PDS).

Pharmaceutical Technology
DECEMBER 5, 2022
With the establishment of this Translational Science Hub, Queensland is set to become an international messenger ribonucleic acid (mRNA) vaccine hub. With an initial focus on enchaining mRNA technology and developing a vaccine for Chlamydia, the research at the hub is anticipated to commence in the first quarter of next year.
Pharmaceutical Technology
SEPTEMBER 29, 2022
The mRNA-1273.222 vaccine comprises 25µg doses of mRNA-1273 (Spikevax) and a vaccine candidate against the BA.4/BA.5 Moderna has so far obtained authorisations for Omicron-targeting bivalent booster vaccine in the US, Canada, Europe, Australia, South Korea, Switzerland, Japan, Singapore, Taiwan and the UK.
Pharmaceutical Technology
OCTOBER 18, 2022
Moderna has entered an agreement with Gavi, the Vaccine Alliance, with regard to the delivery of Covid-19 vaccines to lower-income countries supported by the Gavi COVAX Advance Market Commitment (AMC). According to the deal, the parties will cancel pending orders under the existing Covid-19 vaccine supply agreement for this year.
Pharmaceutical Technology
AUGUST 30, 2022
Moderna has obtaine d temporary authorisation from Swissmedic for its Omicron-targeting bivalent booster vaccine for Covid-19, Spikevax Bivalent Original/Omicron (mRNA-1273.214), for individuals aged 18 years and above. . The vaccine is indicated for active immunisation for the prevention of Covid-19.
World of DTC Marketing
AUGUST 10, 2022
While trust in the pharma industry got a bump because of COVID vaccines, confidence about new drugs remains mixed. Monitor social media and identify possible topics for new content. DTC pharma marketers must acknowledge that people’s days of seeing a TV ad and running to their doctor to ask for the product are all but gone.

World of DTC Marketing
APRIL 14, 2022
“Were they vaccinated?”, In reviewing social media topics and comments on specific health conditions for clients, I determined that more than 75% of posts contained wrong or inaccurate information. One poset said that he had lost his mother and father to Covid, and immediately the trolls weighed in.

Pharmaceutical Technology
OCTOBER 26, 2022
SK bioscience has signed a new collaboration agreement with the Coalition for Epidemic Preparedness Innovations (CEPI) to develop messenger ribonucleic acid (mRNA) vaccines for infectious diseases. The company has obtained the newest vaccine platform technologies such as bacterial culture, cell culture and genetic recombination.
Pharmaceutical Technology
JUNE 1, 2023
A new intracellular drug delivery centre will be established in the UK to support potential ribonucleic acid (RNA) vaccines and therapeutics , as well as the development of innovative drug delivery technologies. Topic sponsors are not involved in the creation of editorial content.
Pharmaceutical Technology
MARCH 23, 2023
China’s National Medical Products Administration (NMPA) has granted emergency use authorisation (EUA) for CSPC Pharmaceutical Group’s messenger RNA (mRNA) vaccine, SYS6006, to treat Covid-19. With this regulatory approval, CSPC Pharmaceutical is claimed to be the first company to receive approval for providing an mRNA vaccine in the country.
Pharmaceutical Technology
AUGUST 23, 2022
5-adapted bivalent vaccine for Covid-19 in people aged 12 years and above. 1-adapted vaccine. The FDA also sought the vaccine’s pre-clinical and manufacturing data for addressing the SARS-CoV-2 virus’ evolution. 5-adapted bivalent vaccine will be made readily available for shipping. 5-adapted bivalent vaccine.

Pharmaceutical Technology
AUGUST 17, 2022
These programmes will include therapies and vaccines in infectious disease and oncology areas. Preclinical data showed the potential of oRNA expression and delivery as a method for advanced development in various areas, including vaccines and oncology treatments. Topic sponsors are not involved in the creation of editorial content.
Pharmaceutical Technology
NOVEMBER 30, 2022
in a project agreement from the US government for developing self-amplifying RNA (saRNA) vaccine technology against advanced and emergent viral threats. Development of vaccines to Phase I trials under the five-year $59m prototype project comprises additional $28.4m Topic sponsors are not involved in the creation of editorial content.
pharmaphorum
OCTOBER 6, 2022
The new version – dubbed Florence 2.0 – covers a broader range of topics. Along with advice on COVID-19 vaccines and treatments it can also share advice on mental health, give tips to de-stress, provide guidance on how to eat healthily and be more active, and quit tobacco and e-cigarettes, according to the WHO.
Pharmaceutical Technology
NOVEMBER 21, 2022
This will aid in the production of new raw materials and possible clinical-grade assets to develop mRNA vaccines and therapies for infectious diseases, as well as other ailments with unmet needs. RVAC is currently developing mRNA-based vaccines against infectious diseases such as Covid-19.

Pharmaceutical Technology
NOVEMBER 7, 2022
5 Omicron-targeting bivalent Covid-19 booster vaccine, mRNA-1273.222, for people aged 18 years and above. The 50µg vaccine is indicated for usage as a booster shot for active immunisation against Covid-19. Additionally, 28 days following inoculation, this vaccine showed enhanced neutralising antibody responses against Omicron BA.4/BA.5
Pharmaceutical Technology
AUGUST 24, 2022
5 Omicron-targeting bivalent booster vaccine for Covid-19. The submission is made for a 50µg booster dose of the vaccine for usage in adults aged 18 years and above. Irrespective of previous infection or age, the vaccine also showed neutralising antibody responses against the BA.4 1 subvariant.

Pharma Marketing Network
DECEMBER 6, 2021
NEW YORK, December 6 th , 2021 (PRNEWSWIRE) – Tap Native, the leading health-focused content discovery platform, today unveiled a list of the top 20 most popular health topics viewed by consumer audiences in 2021. million unique URLs across the top 100 largest health websites in its network to determine which topics were viewed most.

Pharmacy Times
AUGUST 19, 2022
Ed Cohen, PharmD, FAPhA, drives a discussion around the new inclusion of infants as young as 6 months old within the COVID-19 vaccine recommendations and how to educate parents on the topic.

PharmaTech
AUGUST 31, 2022
Dearth of fill/finish capacity in the US, exacerbated by Covid and vaccine production, caused market dislocation but also opportunity. fast-growing, end-to-end CDMO service provider in Nashville, TN that offers development, testing and fill/finish cGMP manufacturing for sterile injectable and topical products.

Pharmaceutical Technology
MAY 12, 2023
BioNTech has ended its research collaboration with Matinas after its oral mRNA vaccine failed to demonstrate preclinical activity. Whilst BioNTech has shelved its oral mRNA vaccine project, shares were up this week following promising early results of a personalised mRNA vaccine for pancreatic cancer.

PM360
MARCH 22, 2024
We’re featuring AI in this month’s issue as the topic can’t be ignored—generative AI is creating huge waves in the Pharma industry and it’s all we’re talking about. Vaccines are being made more quickly than ever with the help of algorithms and you may have even already asked an AI-powered chatbot about a medical condition or concern.
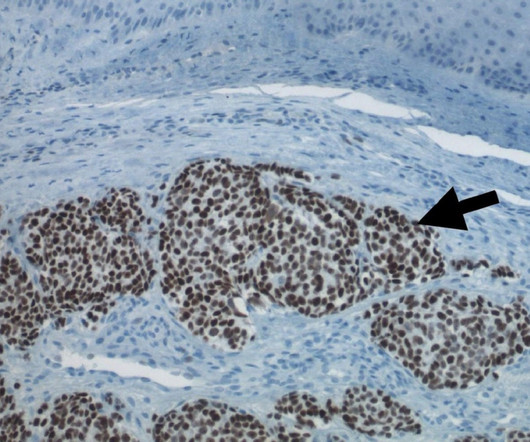
Pharmaceutical Technology
MAY 24, 2023
Morphogenesis’s technologies include Immune Fx (IFx) personalised cancer vaccines and tumour microenvironment (TME) modulators. Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, Morphogenesis’ lead personalised cancer vaccine, IFx-Hu2.0, The company is also advancing its mRNA vaccine, IFx-Hu3.0,

World of DTC Marketing
DECEMBER 28, 2020
First, let’s dispel a recent topic: telehealth. Once vaccination levels reach critical mass, people will once again return to their physician’s offices. Business magazines are writing about the “digital future” of healthcare but are consumers ready? Some are, but most aren’t.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content