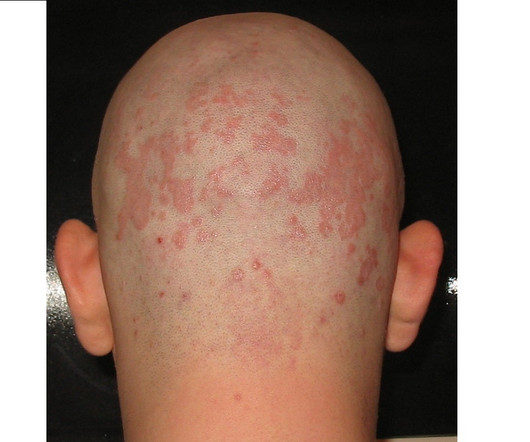
With Vtama's success in relieving itching, Dermavant eyes FDA filing in atopic dermatitis
Fierce Pharma
OCTOBER 11, 2023
In the race to develop nonsteroidal topical treatments for autoimmune skin conditions, Dermavant is charging toward the finish line—again. The trial results bolster Dermavant's effort to win a label expansion for the drug. The trial results bolster Dermavant's effort to win a label expansion for the drug.




































Let's personalize your content