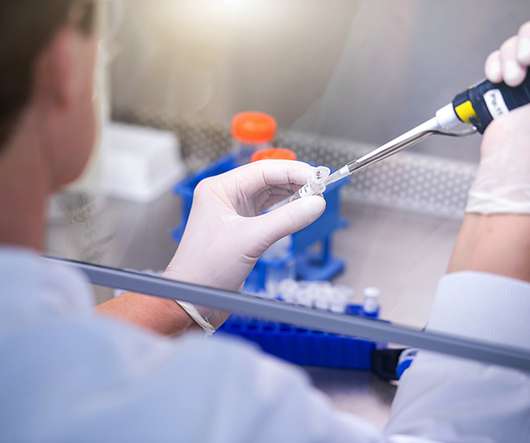
Why is microbiological testing in medical device manufacturing important?
European Pharmaceutical Review
JUNE 15, 2023
This approach can also help determine the appropriate manufacturing processes, sterilisation methods, and maintenance procedures. However, the safety and efficacy of these devices can be compromised if they are not appropriately designed, manufactured, and maintained, the authors wrote. Sharma et al.




































Let's personalize your content