TOP 10 CHALLENGES IN PHARMACEUTICAL PRODUCT LIFE CYCLE MANAGEMENT
eMediWrite
OCTOBER 3, 2022
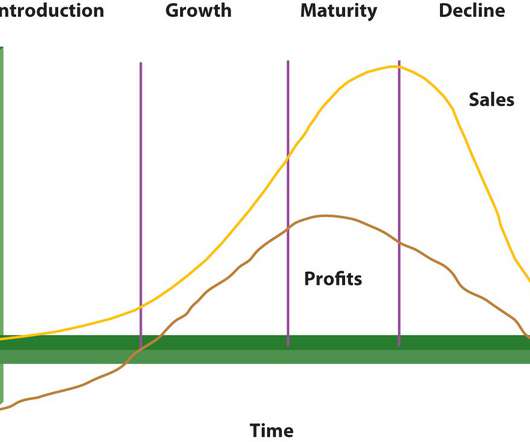
The following are the main obstacles to pharmaceutical product lifecycle management that I have attempted to highlight in this article: Internal and external complexity are rising. Integrated quality and risk management. Registering products globally. Complex collaborative outsourcing networks management.














































Let's personalize your content