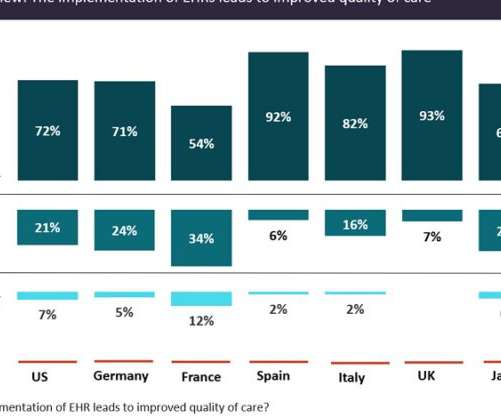
Implementation of electronic health records will improve the quality of care
Pharmaceutical Technology
AUGUST 2, 2022
With more healthcare facilities transitioning from paper charts to electronic records and electronic health records (EHRs) becoming more integrated and technologically advanced, EHRs are expected to drive big changes in the provision of healthcare and clinical research delivery.
















































Let's personalize your content