FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy
Fierce Pharma
MAY 31, 2023
FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy kdunleavy Wed, 05/31/2023 - 13:02
Fierce Pharma
MAY 31, 2023
FDA warns patients about compounded versions of Novo Nordisk's Ozempic, Wegovy kdunleavy Wed, 05/31/2023 - 13:02

MedCity News
MAY 29, 2023
While retailers already have a customer service focus, there is a steep learning curve to healthcare and the nuances of how things operate. It will be critical that these brands do their due diligence and invest in innovations that work to improve the fundamentals of healthcare such as efficient provider credentialing and flexible and sustainable working practices for providers.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
MAY 31, 2023
The inventory position of 28 major pharmaceutical manufacturers in 2022 has been revealed in an annual report. Analysis explored the impact of macro-trends on inventory management from corporate reports of Big Pharma companies including Merck, AstraZeneca and Pfizer. It investigated how excessive inventories are affecting the industry and what factors could influence inventory growth in 2023.
Pharmaceutical Technology
MAY 30, 2023
SK Bioscience has received marketing authorisation from the UK’s medicines and healthcare products regulatory agency (MHRA) for its Covid-19 vaccine, SKYCovion. SKYCovion is intended for the prevention of Covid-19 resulting from SARS-CoV-2 infection in individuals aged 18 years and above. The authorisation allows the distribution of the vaccine in Scotland, Wales and England.

Fierce Pharma
MAY 31, 2023
Adding Kisqali to endocrine therapy after surgery reduced the risk of invasive tumor recurrence or death by 25% in certain early-stage breast cancers.

MedCity News
MAY 29, 2023
While these are key benefits of digital therapeutics and this optimism may have been laudable, this approach to market relies on promise without proof of efficacy and ignores the fact that not all digital therapeutics are created equal.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 31, 2023
XtalPi has made a partnership deal with US-based Eli Lilly to leverage artificial intelligence (AI) for drug discovery. XtalPi will receive $250m in upfront and milestone payments. The two companies will use XtalPi’s integrated AI capabilities and robotics platform for the de novo [without consideration of previous instances] design and delivery of drug candidates for an undisclosed target.

Fierce Pharma
JUNE 1, 2023
As doctors weigh various immunotherapy strategies for the treatment of early-stage non-small cell lung cancer, Merck & Co. | As doctors navigate the "messy" early-stage non-small cell lung cancer landscape with various immunotherapy approaches, Merck & Co. has now unveiled new data that it hopes can establish Keytruda, used before and after surgery, as a new standard of care.

MedCity News
JUNE 1, 2023
Pfizer’s Abrysvo is now approved for preventing illness caused by respiratory syncytial virus, or RSV, in adults 60 and older. The regulatory decision comes weeks after GSK won approval for its RSV vaccine, Arexvy.

PharmaTech
JUNE 2, 2023
An increase in applications for gene therapies is putting stress on FDA’s resources.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
MAY 30, 2023
According to GlobalData’s Looking Ahead to 2023 – the Future of Pharma report, five drugs set for approval in 2023 are projected to attain blockbuster status or near-blockbuster status by 2028 with US company dominance. Lecanemab by Eisai Co Ltd, a monoclonal antibody therapy marketed for Alzheimer’s disease, is predicted to achieve the most significant commercial debut.

Fierce Pharma
JUNE 2, 2023
Lynparza may be the most popular PARP inhibitor, but a use restriction in ovarian cancer has been a thorn in AstraZeneca’s side. | AstraZeneca is bringing on Imfinzi to potentially help Lynparza reach a broader ovarian cancer population. But the lack of evidence for contribution from Imfinzi and a regulatory concern over the use of PARP inhibitors outside BRCA-mutant tumors could make it hard for AstraZeneca to win over the FDA.

MedCity News
JUNE 2, 2023
Digital maturity will require shared data standards, strategies for streamlining data management and a collaborative approach that involves all players in the biopharma supply chain.

Referral MD
MAY 30, 2023
Photo by Marek Studzinski on Unsplash The medical billing process can be a tedious, lengthy, and complex one. This can leave healthcare practitioners struggling to stay afloat financially. Can a healthcare practice increase revenue by improving its medical billing process? A reliable medical billing service or software can help reduce backlogs and increase revenue.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr
Pharmaceutical Technology
JUNE 2, 2023
The European Commission (EC) has approved TG Therapeutics’ Briumvi (ublituximab-xiiy) to treat relapsing forms of multiple sclerosis (RMS) in adult patients. Briumvi is an anti-CD20 monoclonal antibody indicated for RMS adult patients with active disease which is defined by clinical or imaging features. It has been engineered to remove certain sugar molecules generally expressed on the antibody.
Fierce Pharma
MAY 30, 2023
Alnylam sticks with aggressive litigation strategy against Pfizer and Moderna, filing yet another patent lawsuit zbecker Tue, 05/30/2023 - 10:24

MedCity News
JUNE 1, 2023
Recent regulations from the Office of the National Coordinator for Health IT (ONC) and Centers for Medicare & Medicaid Services (CMS) have, respectively, focused on prohibiting information blocking and increasing price transparency. Together, these two regulations have put a third issue into the spotlight as well: Reducing the complexity of clinical terminology.
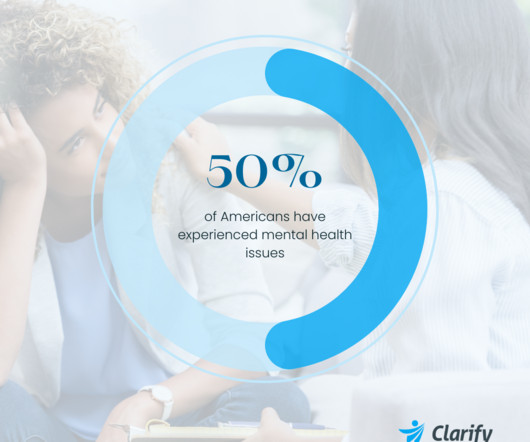
Clarify Health
MAY 30, 2023
Addressing the mental health crisis in the United States is a complex undertaking, due to multiple factors contributing to prevalence and severity of several conditions. Recent findings from the Clarify Health Institute highlight these growing problems and speak to a need for action to combat this crisis. According to its most recent research brief, “The Kids Are Not Alright: Mental Health Utilization Among Children and Young Adults, 2016-2022,” hospital admissions for mental illnes

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmaceutical Technology
JUNE 1, 2023
The UK’s National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending the use of Pfizer ’s rimegepant (Vydura) to prevent migraine attacks. The therapy has been recommended as an option to prevent episodic migraines in adult patients, who have between four and 15 migraine attacks per month, and where a minimum of three preventive treatments have previously failed.

Fierce Pharma
JUNE 1, 2023
After a panel of independent experts endorsed a narrow approval for AstraZeneca and Merck’s Lynparza in metastatic castration-resistant prostate cancer (mCRPC), the FDA has followed suit. | The drug is now approved to treat BRCA-mutated metastatic castration-resistant prostate cancer in combination with Johnson & Johnson’s Zytiga and a corticosteroid.

MedCity News
JUNE 1, 2023
A new decentralized network of the future exists and is in operation today. Large payers and providers are already beginning to use it for administrative transactions, although clinical transactions are also envisioned.

European Pharmaceutical Review
JUNE 2, 2023
A novel refractometry-based process analytical technology (PAT) system used to monitor HEK293T cell cultures during lentiviral vector (LVV) production in real time, was able rapidly identify a relationship between bioreactor pH and culture metabolic activity, a paper has found. Using PAT in the biotechnology and biopharmaceutical industries is a radical vision” Williams et al. stated that to their knowledge, the study represents the first time that pH has been incorporated into a metabolic

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
MAY 29, 2023
Lexicon Pharmaceuticals (Lexicon) has received approval from the US Food and Drug Administration (FDA) for its Inpefa drug to treat heart failure. Inpefa is a once-daily oral tablet indicated as an inhibitor of sodium-glucose co-transporter type 2 (SGLT2) and type 1 (SGLT1). It is intended to lower the risk of cardiovascular death, urgent heart failure visit, and hospitalisation for heart failure.

Fierce Pharma
MAY 31, 2023
After a yearslong investigation involving politicians and medical professionals alike, Novartis is looking to close the books on its Greek bribery imbroglio. | After an investigation that dates back to 2017, four former Novartis executives and a Greek politician were cleared of bribery charges. But 15 doctors were charged for their alleged involvement.

MedCity News
MAY 29, 2023
Ray Therapeutics’ gene therapy is independent of the causative genes driving inherited vision disorders, which CEO Paul Bresge says is important for reaching a broad patient population. Rare disease retinitis pigmentosa is the first disease target, but the startup also plans to test its approach in more prevalent eye disorders.

European Pharmaceutical Review
MAY 29, 2023
A neurosteroid drug was one of two medicines recommended for approval at the Committee for Medicinal Products for Human Use (CHMP) ’s May 2023 meeting. Ztalmy (ganaxolone) received a positive opinion for epileptic seizures associated with cyclin-dependent kinase-like 5 deficiency disorder. This genetic condition is defined by seizures starting during infancy.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharmaceutical Technology
MAY 31, 2023
Stealth BioTherapeutics has made an exclusive licensing agreement with Pharmanovia for the commercialisation of its elamipretide to treat Barth syndrome. Pharmanovia will hold exclusive rights for the commercialisation of elamipretide in the EU, other European countries including Switzerland, Norway, Iceland and the UK, and the Middle East and northern Africa region.

Fierce Pharma
JUNE 1, 2023
About a month after GSK won the world's first approval for a respiratory syncytial virus (RSV) vaccine, rival Pfizer has followed suit. | After winning FDA approvals one month apart, Pfizer and GSK are set to launch competing RSV vaccines this fall.

MedCity News
MAY 30, 2023
Employers have a responsibility to support the food as medicine movement and help employees who are food insecure, said Jay Bhatt, managing director of Deloitte Services and managing director of the Center for Health Solutions and Health Equity Institute. He made these comments last week at the MedCity INVEST conference in Chicago.

PharmaTech
JUNE 1, 2023
A partnership between Rentschler Biopharma and Ikarovec will support the accelerated development of novel gene therapies for the treatment of eye diseases.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content