Debunking Healthcare’s Recession-Proof Reputation
MedCity News
AUGUST 31, 2023
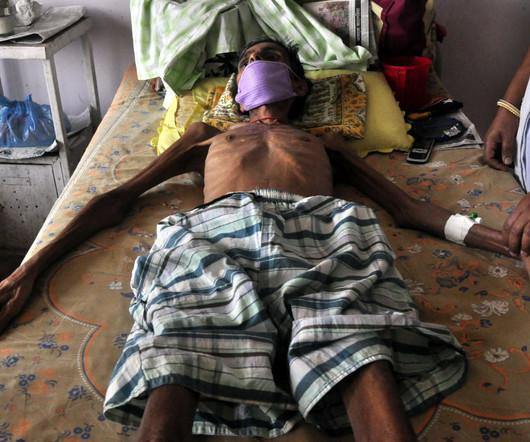
During past recessions, the healthcare sector remained relatively immune to economic downswings — but things are different now that a sweeping labor shortage and lower patient volumes have been added to the mix.





































Let's personalize your content