FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists
Fierce Pharma
MAY 16, 2023
FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists fkansteiner Tue, 05/16/2023 - 09:33
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Fierce Pharma
MAY 16, 2023
FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists fkansteiner Tue, 05/16/2023 - 09:33

Rep-Lite
NOVEMBER 30, 2023
B2B sales consulting for medical sales is a specialized service that helps healthcare organizations navigate the unique challenges of B2B transactions within the healthcare industry. What is B2B Sales Consulting? B2B sales consulting involves working with businesses to enhance their sales strategies and operations in a B2B context.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
NOVEMBER 2, 2023
The US Food and Drug Administration ( FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to Johnson & Johnson’s Stelara (ustekinumab). The evidence also demonstrated that Wezlana met the other legal requirements to be interchangeable with Stelara at the pharmacy level, without consulting the prescriber.
Legacy MEDSearch
OCTOBER 6, 2022
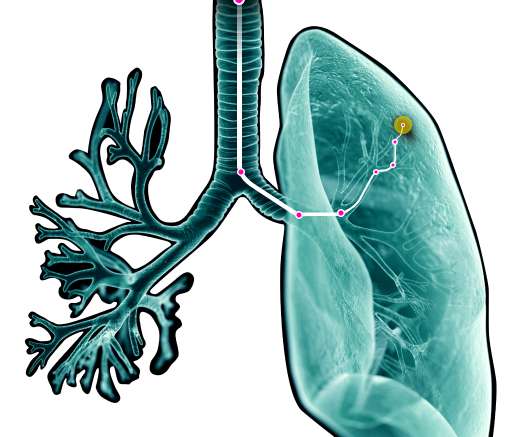
Body Vision Medical, a leader in AI-driven, intraoperative imaging, announced a new partnership with Business Asia Consultants (BAC), Inc. Body Vision Medical’s LungVision system was FDA cleared in 2019 and received CE Mark in 2021. About Business Asia Consultants. Business Asia Consultants (BAC), Inc. Partnership.

European Pharmaceutical Review
NOVEMBER 28, 2022
Fast Track designation (FTD) has been granted for REM-001 therapy to treat unresectable cutaneous metastatic breast cancer (CMBC) by the US Food and Drug Administration (FDA). The post FDA Fast Track designation for photodynamic cancer therapy appeared first on European Pharmaceutical Review.

pharmaphorum
JULY 21, 2022
Now FDA on-site inspections have resumed, with regulatory authorities returning to physical sites. Throughout the pandemic, the lack of FDA inspections has created new challenges and heightened existing problems, largely due to delays in upgrading legacy facilities. Upgrading facilities. Ensuring GMP readiness.

PharmaTech
SEPTEMBER 19, 2023
Maik Jornitz, Principal Consultant, BioProcess Resources LLC, discusses the definition of patient safety and how to implement new technologies into upgraded facilities.

European Pharmaceutical Review
MAY 9, 2024
This order directed the three agencies to improve their implementation of The Coordinated Framework for the Regulation of Biotechnology, FDA stated. FDA highlighted that the collaborators’ plan also meets another of the President’s goals: ensuring that the public is confident in the biotechnology regulatory system.
Pharmaceutical Technology
APRIL 11, 2023
Oracle company Cerner Enviza and John Snow Labs have collaborated with the US Food and Drug Administration (FDA) for the development of artificial intelligence (AI) tools for drug safety and real-world evidence studies. Together, Cerner Enviza and John Snow Labs have all the right expertise, data and technology to make it happen.”

Pharmaceutical Technology
NOVEMBER 7, 2023
Patients who have used the products should consult their healthcare provider and properly discard the product.
Pharmaceutical Technology
NOVEMBER 2, 2022
Ascletis Pharma has submitted an Investigational New Drug (IND) application to the US Food and Drug Administration (FDA) for its drug candidate for Covid-19. The company made the submission after the pre-IND consultation with the regulatory agency. An oral small molecule inhibitor, ASC11 acts on 3-chymotrypsin like protease (3CLpro).

Legacy MEDSearch
MAY 30, 2023
Food and Drug Administration (FDA). THINK Surgical designed the TMINI robotic handpiece in collaboration with Sagentia Innovation, an R&D consultancy based in Cambridge, U.K. About Sagentia Innovation Sagentia Innovation is a science and engineering R&D consultancy based in Cambridge, U.K. THINK Surgical, Inc.,

Legacy MEDSearch
AUGUST 22, 2022
Food and Drug Administration (FDA) has granted 510(k) clearance to the MedWand,” shared MedWand CEO and Co-Founder, Robert Rose. “As With FDA clearance, clinicians and patients everywhere can trust MedWand’s vitals and remote examination tools, and experience telemedicine on an entirely new level.” MedWand Solutions, Inc.

PharmaTech
SEPTEMBER 21, 2023
Maik Jornitz, Principal Consultant, BioProcess Resources LLC, discusses how to upgrade facilities in an efficient manner and other highlights from his presentation.
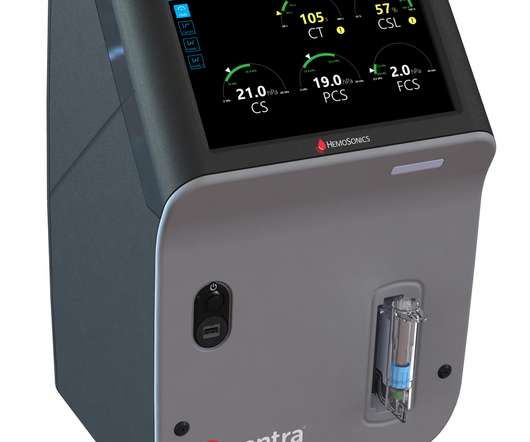
Legacy MEDSearch
DECEMBER 1, 2022
Food and Drug Administration (FDA) for the Quantra Hemostasis System with QStat Cartridge. The FDA clearance of the QStat Cartridge expands the Quantra System’s indications for use to include trauma, and liver transplantation procedures. market today. “Point-of-care data is the answer to PBM-guided patient decisions.
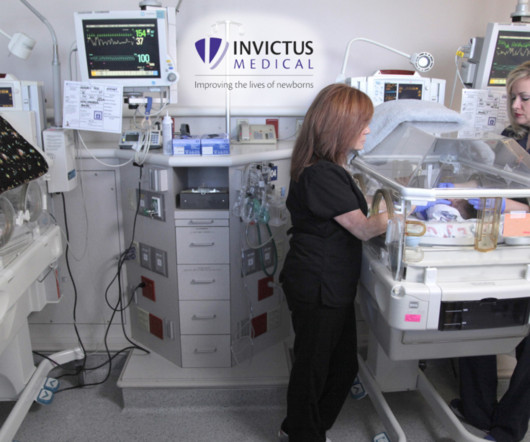
Legacy MEDSearch
JULY 11, 2023
Invictus Medical announced today that the culmination of their De Novo application to the FDA for its Neoasis ® incubator-based active noise control (ANC) device has resulted in a clearance-for-use declaration by the FDA. Invictus Medical is a privately held company. Are you hiring?

European Pharmaceutical Review
MARCH 5, 2024
The US Food and Drug Administration (FDA) recently highlighted data integrity issues concerning premarket submissions received for medical devices. Consequently, because “it calls into question the data integrity of the entire file”, FDA asserted that is unable to rely on the data to grant marketing authorisation.

Legacy MEDSearch
SEPTEMBER 22, 2022
Food and Drug Administration (FDA) has granted QT Imaging, Inc. No other ultrasound-based breast imaging modality is cleared by the FDA to quantify fibroglandular volume. ” FDA K220933. The company has received FDA 510(K) clearance for its QT Imaging Breast Scanner. About QT Imaging, Inc.

pharmaphorum
JULY 28, 2022
That means maintaining regulatory readiness – because having cGMP compliance problems with the FDA can significantly exacerbate the problem. If a company’s submission to the FDA refers only to suppliers from China, it is no small feat to resubmit an application for an alternative supplier elsewhere. About the Author.

pharmaphorum
SEPTEMBER 29, 2022
Food and Drug Administration (FDA) regulation as a medical device. Determining whether a product is a medical device subject to FDA regulation necessarily begins with understanding the FDA regulatory definition of ‘medical device’. When assessing mHealth technology, the FDA will determine whether an application is either: i.

Pharmaceutical Technology
JANUARY 31, 2023
FDA director Robert Califf gave his prognosis for the pharma industry at this year’s JP Morgan Healthcare Conference in San Francisco over January 9–12. asked panel chair Ipsita Smolinski, MD of consultancy Capitol Street. This “skinny” bill passed just in time to continue funding the FDA, with few of the planned amendments.

pharmaphorum
JULY 19, 2022
Speaking during a Patients as Partners meeting, Ebony Dashiell-Aje, senior director of patient engagement and outcomes research at BioMarin Pharmaceuticals, said these small, informal, non-regulatory events helped the FDA “better understand what is most important” among specific patient groups. The rise of patient centricity. “We
European Pharmaceutical Review
APRIL 30, 2024
Last week, the US FDA approved a new gene therapy for eligible adults with haemophilia B. A positive opinion of a marketing authorisation was granted for Altuvoct (efanesoctocog alfa), as a treatment and prophylaxis of bleeding in patients with the rare blood disorder haemophilia A.

Medical Device Success
MAY 30, 2021
Now he is a consultant for both healthcare systems and some of the largest life science companies in the world. Now he is a consultant for both healthcare systems and some of the largest life science companies in the world. How will the Four Forces shaping healthcare affect your sales and marketing strategies and tactics?

European Pharmaceutical Review
NOVEMBER 27, 2023
According to the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) , Kanuma is approved as an LAL-D treatment in countries such as the US, EU, Japan, Canada and other countries. Evidence of successful long-term management of the disease was reported in a case study published last year in the Canadian Liver Journal.

European Pharmaceutical Review
MARCH 4, 2024
Indeed, in 2022, biologics constituted 40 percent of all US Food and Drug Administration (FDA) approved drugs, projecting a compound annual growth rate (CAGR) of 9.5 The emergency use approval from the FDA of lipid nanoparticle- (LNP-) based mRNA vaccines during the COVID-19 pandemic attested the significance of LNPs.

Legacy MEDSearch
OCTOBER 13, 2022
Food and Drug Administration (FDA) for the Senza HFX iQ spinal cord stimulation (SCS) system. The company encourages investors and potential investors to consult the Nevro website regularly for important information about Nevro. Senza HFX iQ is the first and only Artificial Intelligence-based SCS system that learns from patients.

European Pharmaceutical Review
FEBRUARY 22, 2023
1 Consequently, the US Food and Drug Administration (FDA) and other agencies are keen to see “accelerated development” programmes in areas where there is a significant unmet clinical need. He is currently a CMC consultant with an interest in impurities and safety‑based limits. FDA; 2018 [cited 2023Jan]. billion, and rising.

European Pharmaceutical Review
MARCH 5, 2024
Visitors can gain insights into compliance with industry-specific validation guidelines and requirements such as GMP , GLP, REACH and FDA. Well-known manufacturers provide information on current developments in refrigeration technology and offer green consultations for laboratories. These enable sustainable cooling in the laboratory.

European Pharmaceutical Review
NOVEMBER 27, 2023
According to the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) , Kanuma is approved as an LAL-D treatment in countries such as the US, EU, Japan, Canada and other countries. Evidence of successful long-term management of the disease was reported in a case study published last year in the Canadian Liver Journal.

PM360
SEPTEMBER 7, 2023
Digital insights consultancy Creation Healthcare gathered sentiments of HCPs about changes to X (Twitter) and transferring to different platforms, like Meta’s Threads. Doctor Docs: HCPs on Social Media Platforms X vs. Threads Reaching HCPs where they prefer to consume content isn’t as simple with recent changes to the social media landscape.

pharmaphorum
SEPTEMBER 30, 2022
This means that drugs such as Keytruda, which has received almost 40 FDA approvals since its launch, can offer significant volume-based rebates. CRA is a leading global consulting firm that offers strategy, financial, and economic consulting services to industry, government and financial clients. About the authors.

Pharma Marketing Network
MARCH 23, 2023
The Food and Drug Administration (FDA) has recently approved a new drug for the treatment of Alzheimer’s disease called Leqembi. In recent years, several drugs for Alzheimer’s have been developed, but none have been able to gain approval from the FDA. FDA Approves Leqembi for the Treatment of Alzheimer’s Disease.

European Pharmaceutical Review
AUGUST 28, 2023
This authorisation was granted by the US Food and Drug Administration (FDA). On 22 December 2021, Leqvio (inclisiran) was the first siRNA therapeutic to be approved to reduce LDL-C. The post siRNA therapy shows long-term potential in lowering LDL-C appeared first on European Pharmaceutical Review.

Pharma Marketing Network
MAY 3, 2023
The United States Food and Drug Administration (FDA) is the primary regulatory agency that oversees the approval, promotion, and advertising of drugs. The FDA has strict guidelines for what information can be included in drug advertisements and how it should be presented to the public.

pharmaphorum
NOVEMBER 29, 2022
In the coming weeks, Genentech will work with the FDA to complete the withdrawal process and notify healthcare professionals (HCPs). Patients given the Tecentriq plus chemotherapy combination survived a median of 16 months after treatment, compared with 13.4 months for those receiving only chemotherapy.

Nixon Gwilt Law
JANUARY 13, 2022
This overview is designed to help you understand what to expect, how to prepare, and why you don’t want to rush the process, take shortcuts, or overlook the importance of establishing a good relationship with the FDA. What FDA approval pathways are available for medical devices? What happens in a pre-submission meeting?

Clarivate
OCTOBER 19, 2023
the FDA offers a period of 3 years of data exclusivity for a new application of a previously approved drug. Computational methods range from molecular docking and binding-site detection to pathway mapping, genetic associations and machine learning (ML). For example, in the U.S.,

MedCity News
JULY 27, 2023
In February of 2019, Johnson & Johnson acquired Auris Health and its FDA-cleared Monarch platform for $5.75 Later in 2019, Germany-based Siemens Healthineers announced its acquisition of Corindus and its FDA-cleared CorPath GRX System, a robotics platform specifically for percutaneous coronary interventions.

World of DTC Marketing
MARCH 8, 2021
Whether it’s through their broker, insurance company, or consultants, businesses should examine these costs closely and understand where they are deviating from benchmarks and why. The FDA could require all pharma product websites to add general health prevention information and links to credible online health information.

European Pharmaceutical Review
AUGUST 3, 2023
US Food and Drug Administration (FDA) guidance on medical device quality defines “least burdensome” to be the minimum amount of information necessary to adequately address a relevant regulatory question or issue through the most efficient manner at the right time, the MDIC highlighted.

Pharma Marketing Network
MAY 11, 2023
Food and Drug Administration (FDA) in June 2021. Aduhelm is the first Alzheimer’s drug to receive FDA approval in nearly two decades, and it works by clearing the sticky amyloid plaques that accumulate in the brains of Alzheimer’s patients. 2021, June 7). Retrieved from [link] · What is Alzheimer’s disease?

LEVO Health
OCTOBER 26, 2022
Food & Drug Administration (FDA) classifies medical devices. How the FDA Classifies Medical Devices. The FDA classifies medical devices into one of three classes based on their risks and regulatory controls: . The FDA classifies medical devices into one of three classes based on their risks and regulatory controls: .

pharmaphorum
SEPTEMBER 29, 2022
A digital patient monitoring tool developed by UK company CyberLiver has been given a breakthrough designation by the FDA for out-of-hospital management of patients with cirrhosis at risk of complications. The post Digital tool cuts hospital readmissions in cirrhosis patients appeared first on.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content