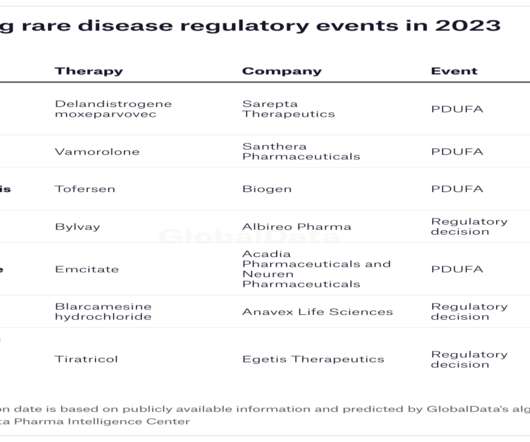
FDA delays decision on Biogen’s ALS hope tofersen
pharmaphorum
OCTOBER 17, 2022
The FDA is planning to take an additional three months to review Biogen’s experimental therapy for amyotrophic lateral sclerosis (ALS), setting back its decision date from January to April. A few weeks ago the delay would have been a big deal for Biogen, as tofersen was among its most important near-term launch prospects.









































Let's personalize your content