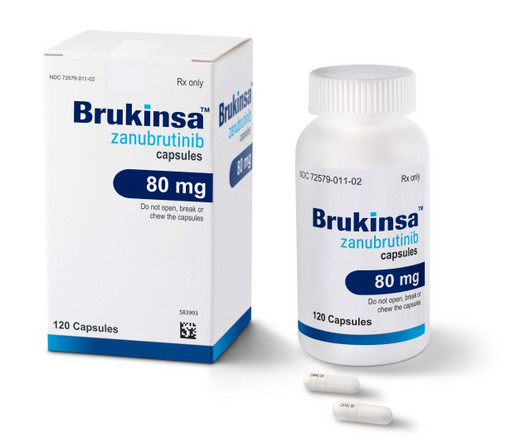
As Imbruvica stumbles, BeiGene's Brukinsa steps up with FDA nod in follicular lymphoma
Fierce Pharma
MARCH 1, 2024
With a new FDA nod, BeiGene has filled the follicular lymphoma approval gap for BTK inhibitors. BeiGene has filled the follicular lymphoma gap for BTK inhibitors with an FDA approval for Brukinsa about a year after AbbVie and Johnson & Johnson's Imbruvica stumbled in a phase 3 trial.






































Let's personalize your content