Cannabinoids show promise in acute migraine clinical trial
pharmaphorum
MARCH 4, 2024
Inhaled cannabinoids have been shown to perform better than placebo in providing pain relief for people suffering from acute migraine in a clinical trial
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical migraine
clinical migraine 
pharmaphorum
MARCH 4, 2024
Inhaled cannabinoids have been shown to perform better than placebo in providing pain relief for people suffering from acute migraine in a clinical trial

European Pharmaceutical Review
SEPTEMBER 14, 2023
Rimegepant (Vydura) is the “first and only NICE-recommended medicine that can help alleviate the misery of acute migraines, and may be considered a step-change in treatment,” shared Helen Knight, Director of medicines evaluation at the National Institute for Health and Care Excellence (NICE).
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
NOVEMBER 17, 2023
Results from AbbVie’s Phase III study, PRODROME , published in The Lancet showed that UBRELVY ® (ubrogepant) 100mg for acute treatment of migraine significantly reduced the likelihood of development of moderate or severe headache compared to placebo within 24 hours post-dose.

Pharmaceutical Technology
JUNE 1, 2023
The UK’s National Institute for Health and Care Excellence (NICE) has issued final draft guidance recommending the use of Pfizer ’s rimegepant (Vydura) to prevent migraine attacks. NICE medicines evaluation director Helen Knight said: “Each year, the lives of millions of people in England are blighted by migraine attacks.

European Pharmaceutical Review
AUGUST 17, 2023
Recent EU approval means adults who have four or more migraine days per month can now access the first and only once-daily oral calcitonin gene-related peptide (CGRP) receptor antagonist (gepant). This was compared to 27 percent of patients in the placebo arm. This result was compared to 29 percent of participants given placebo.

Legacy MEDSearch
DECEMBER 12, 2022
Theranica , a prescribed digital therapeutics company developing advanced neuromodulation devices for migraine and other pain conditions, announced that Highmark Inc. “Nerivio is an excellent treatment option for our patients with migraine, especially those who do not respond to medications, or cannot tolerate their side effects.

Pharmaceutical Technology
MARCH 10, 2023
The US Food and Drug Administration (FDA) has granted approval for Pfizer ’s Zavzpret (zavegepant) for the acute treatment of migraine in adult patients with or without aura. Zavzpret is claimed to be the first and only calcitonin gene-related peptide (CGRP) receptor antagonist nasal spray approved to treat migraine.

European Pharmaceutical Review
JUNE 1, 2023
An oral treatment for preventing migraines has been recommended by the National Institute for Health and Care Excellence (NICE) for the first time. Rimegepant (Vydura) is recommended as an option for episodic migraine in adults after having at least three previous failed preventive treatments. How does the oral migraine treatment work?

Legacy MEDSearch
OCTOBER 25, 2023
Theranica , a neuromodulation therapeutics company, announced the publication of a comprehensive clinical study in Advances in Therapy examining the long-term utilization, clinical efficacy, and safety of Nerivio ® , a Remote Electrical Neuromodulation (REN) device for the treatment of migraine.

Pharmaceutical Technology
OCTOBER 4, 2022
A CGRP receptor antagonist, NURTEC ODT is approved by the US Food and Drug Administration (FDA) for use in adults for acute treatment of migraine irrespective of aura status as well as for preventive episodic migraine treatment. It is also approved in the EU under the trade name Vydura for use in similar indications.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has accepted Satsuma Pharmaceuticals’ 505(b)(2) new drug application (NDA) for STS101 for acute treatment of migraine, for review. It provides significant benefits compared to the current acute treatments for migraine.

Pharmacy Times
JUNE 30, 2023
Galcanezumab-gnlm (Emgality; Eli Lilly and Company) did not demonstrate superiority to rimegepant orally disintegrating tablet (Nurtec ODT) on the percentage of individuals achieving a 50% or greater reduction in monthly migraine headache days.

Pharmaceutical Technology
NOVEMBER 18, 2023
LuAG-09222 is under clinical development by H. Lundbeck and currently in Phase II for Migraine.

Pharmaceutical Technology
FEBRUARY 8, 2024
Prabotulinumtoxin A biosimilar is under clinical development by AEON Biopharma and currently in Phase II for Migraine.

Pharmaceutical Technology
JULY 19, 2022
AbbVie has filed a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) for its atogepant for prophylaxis of migraine in adults who experience a minimum of four migraine days each month. An oral CGRP receptor antagonist (gepant), atogepant is developed as a preventive migraine treatment.

Pharmacy Times
JANUARY 4, 2023
There is a growing body of evidence that foods, substances, and additives in vitamins and supplements can be a trigger for migraine headaches.

pharmaphorum
JULY 18, 2022
AbbVie has submitted its oral CGRP inhibitor atogepant for prevention of both episodic and chronic migraine in the EU as it tries to catch up with main rivals Pfizer/BioHaven and their recently-approved Vydura drug. GlobalData has said it expects Qulipta to get approval for chronic migraine prevention in 2023, helping it to sales of $1.2

PM360
AUGUST 9, 2022
Following the annual American Society of Clinical Oncology (ASCO) conference, oncologists were asked how the data presented would impact their care of patients. When asked about driving diversity in clinical trials, 31% of surveyed oncologists felt the best solution is to create a network of clinical trial sites in underserved communities.
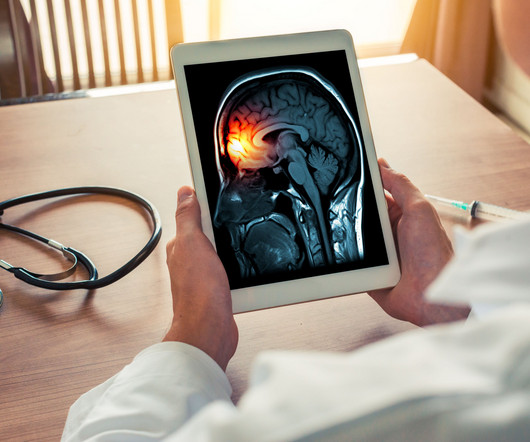
Pharmacy Times
DECEMBER 7, 2023
Amanda Hickman, PharmD, MPH, MSCS, discusses the clinical assessment to lower migraines (CALM) in an HSSP setting.

Clarivate
OCTOBER 31, 2023
billion spend on Biohaven , for example, has brought in a migraine franchise to the pharma major that had Comirnaty cash burning multiple holes in its pockets. That deal brings in a CGRP drug franchise, including the approved therapy Nurtec ODT, the only oral CGRP drug approved to prevent and treat migraines. Pfizer’s $11.6

Pharmaceutical Technology
JUNE 10, 2023
RT-002 is under clinical development by Revance Therapeutics and currently in Phase II for Upper Limb Muscle Spasticity. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is administered by intramuscular route and a unspecified indication.

Pharmaceutical Technology
JUNE 10, 2023
RT-002 is under clinical development by Revance Therapeutics and currently in Phase II for Upper Limb Muscle Spasticity. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is administered by intramuscular route and a unspecified indication.

European Pharmaceutical Review
JULY 18, 2022
Nebido is typically administered by a physician every 10-14 weeks for treatment of male hypogonadism or testosterone deficiency, It can also be used to treat clinical symptoms such as regression of secondary sexual characteristics, change in body composition, asthenia, reduced libido or erectile dysfunction.
European Pharmaceutical Review
JANUARY 11, 2024
To overcome these challenges, there have been efforts in creating more accurate bedside testing, such as eg, Quantitative Sensory Testing, and more emphasis is put on early clinical translational studies. What is the present clinical landscape for pain management medicines, especially chronic pain? References Cruccu G, Truini A.
pharmaphorum
NOVEMBER 11, 2022
The lack of diversity in clinical trials has been a topic of debate for decades, but was thrust into the spotlight as the impact of the pandemic on poorer, less educated and ethnically diverse populations became even more apparent. The post IQVIA’s report card for clinical trial diversity: must do better appeared first on.

Pharmaceutical Technology
NOVEMBER 29, 2022
Researchers are also interested in ketamine’s role for the treatment of migraines. Citing his clinical experience, Dr. Eric Schwenk, professor of anesthesiology and orthopedic surgery at Thomas Jefferson University in Philadelphia, says that there are many people with migraine who would benefit from ketamine.

European Pharmaceutical Review
JULY 13, 2023
1 Europe, however, is well placed to become a leader in driving the clinical translation of medications that display significant promise in laboratory-based studies. Cannabis complications There are notable complexities to researching medical cannabis using a linear approach to clinical translation.

Pharmaceutical Technology
FEBRUARY 27, 2023
Hydrocortisone is under clinical development by Antares Pharma and currently in Phase I for Adrenal Insufficiency. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It also develops products to reduce the risk of preterm birth in pregnant women.

pharmaphorum
AUGUST 8, 2022
Meanwhile, bluebird bio’s one-time gene therapy lovotibeglogene autotemcel is supposed to be heading for regulatory filing in the US next year, although it has been delayed by an FDA partial clinical hold implemented after a persistent case of anaemia was seen in one adolescent patient in a clinical trial.

Pharmaceutical Technology
JULY 20, 2022
Prescribing it] requires knowledge of a patient’s medical history, as well as clinical monitoring for side effects and follow-up care to determine whether a patient is improving—requirements far beyond a pharmacist’s scope and training,” as per a statement by AMA president Jack Resneck.
pharmaphorum
NOVEMBER 15, 2022
Among those who partook in the survey, the most diagnosed chronic conditions they suffered from were anxiety (56%) and depression (52%), and chronic pain (26%), hypertension (24%), and migraine (22%). Of those who were afflicted by anxiety, 73% also battled with depression.

Pharmaceutical Technology
FEBRUARY 20, 2023
According to Globaldata, it is involved in 17 clinical trials, of which 11 were completed, and 6 were terminated. The rNPV model is a more conservative valuation measure that accounts for the risk of a drug in clinical development failing to progress. S,S-Reboxetine is administered through oral route.

pharmaphorum
AUGUST 21, 2022
There’s no word yet on its pricing, but for comparison Spravato was launched with an annual price tag of around $32,400, which the Institute for Clinical and Economic Review (ICER) concluded was around 25% to 52% too high to be cost effective.

Clarivate
APRIL 18, 2024
As Amazon taps technology to rewire healthcare and medicine delivery, employee-owned supermarket chain Hy-Vee is launching new programs that draw consumers to its robust retail health and pharmacy business, which includes a fleet of mobile health units and numerous healthcare clinics operated by provider partners, among other services.

pharmaphorum
NOVEMBER 2, 2022
One company that is applying digital therapeutics to chronic pain is Jogo Health, which has developed solutions for pelvic pain, migraine/tension type headache, and chronic lower back pain. Digital therapeutics can also provide patients with education on their condition and help to maintain motivation to stick with treatment plans.

PM360
JUNE 29, 2022
The migraine therapy market has heated up over the past few years with the emergence of many new acute and preventive medications. Only about half of those living with migraine (52%) have tried acute therapy, and just 37% have investigated preventive therapy, the survey found. Migraine Is Widespread, But Expertise Is Limited.

Clarivate
APRIL 6, 2022
Pre-publication peer review: Can Ciprofloxacin be Used for Precision Treatment of Gonorrhea in Public STD Clinics? Post-publication peer review: Brain state monitoring for the future prediction of migraine attacks. Pre-publication peer review: Patterns and mechanisms in instances of endosymbiont-induced parthenogenesis.

Pharma Marketing Network
JUNE 10, 2020
And it’s the first time I’m seeing our friends on the clinical side of the business out innovating the commercial side of the business in pharma. Novartis, Boehringer, Pfizer are all on the clinical side really chasing the innovations around R&D. Now to get somebody into a clinical trial.

Pharma Marketing Network
JUNE 10, 2020
And it’s the first time I’m seeing our friends on the clinical side of the business out innovating the commercial side of the business in pharma. Novartis, Boehringer, Pfizer are all on the clinical side really chasing the innovations around R&D. Now to get somebody into a clinical trial.

European Pharmaceutical Review
SEPTEMBER 7, 2022
These newly developed tools can be used in clinical research, for instance drug absorption studies, and can contribute to faster and more efficient development of oral pharmaceutical products. For example, capsules have been created that allow for monitoring of external conditions, such as pH, and external control over drug release.

Celeritas
FEBRUARY 20, 2023
Whether setting up a clinic, refreshing your practice, or adding new wings or care outlets, this article will be your guide to organizing your inventory in an efficient and intuitive manner. The variety and complexity of inventory will vary from small, privately owned clinic to vast veterinary hospital chains.

Medico Reach
MAY 10, 2022
The company is known for revolutionary clinical trials and biotechnology research. The key products in their catalog include Aimovig for treating migraine and Corlanor for addressing chronic heart failure. Invention Ranking : 1. Innovation ranking : 2 . Industry revenue : $81.3 CEO : Albert Bourla.
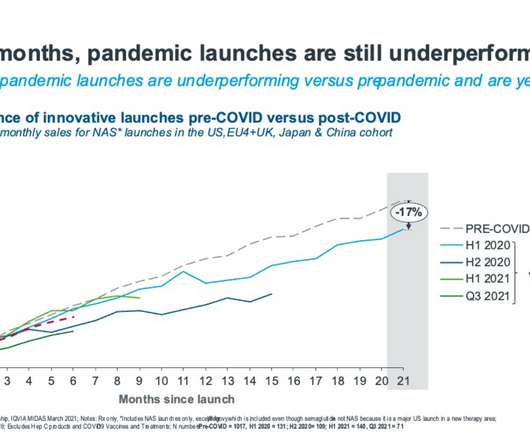
pharmaphorum
JUNE 29, 2022
Kirstie has over 5 years’ experience in healthcare and life sciences, previously working to provide industry expertise and analytics which support industry associations to influence health policy, and providing clinical and commercial competitive insights for pharmaceutical companies.

Pharmaceutical Technology
MAY 10, 2023
On May 9, The Scottish Medicines Consortium approved the inclusion of Pfizer and Biohaven’s Vydura (rimegepant) into the Scottish National Health Services (NHS) for the acute treatment of migraines. However, the regulator did not grant the drug approval for its use in preventing migraines.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content