RAPT hit by FDA clinical hold on eczema, asthma drug
pharmaphorum
FEBRUARY 21, 2024
RAPT Therapeutics shares fell sharply after the FDA placed a clinical hold on oral CCR4 antagonist zelnecirnon in atopic dermatitis and asthma.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical asthma
clinical asthma 
pharmaphorum
FEBRUARY 21, 2024
RAPT Therapeutics shares fell sharply after the FDA placed a clinical hold on oral CCR4 antagonist zelnecirnon in atopic dermatitis and asthma.

Pharmaceutical Technology
MAY 22, 2023
The Center for Drug Evaluation (CDE) of China’s National Medical Products Administration (NMPA) has accepted an investigational new drug application (IND) for SinoMab BioScience’s SM17 to treat asthma. SinoMab BioScience intends to commence a Phase I clinical trial in China to evaluate SM17’s safety profile.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
MedCity News
JULY 12, 2022
New company Areteia Therapeutics launched with up to $350 million in financing and an asthma drug candidate from Knopp Biosciences.

Pharmaceutical Technology
APRIL 26, 2023
AstraZeneca , Teva Pharmaceuticals , and Novartis , and other companies, are expanding their reach in the asthma space by capitalizing on digital technologies to enhance the patient experience. Digital inhalers empower patients to be more proactive in taking control of their asthma,” says Mosnaim.

Pharmacy Times
SEPTEMBER 29, 2023
A workgroup of asthma treatment experts introduced 6 new criteria that those diagnosed with asthma must meet to be in remission.

Pharmaceutical Technology
JANUARY 8, 2023
In 2023, the pharmaceutical industry will mark 20 years since Xolair, an anti-IgE antibody, became the first biologic approved to treat asthma. Since then, the US FDA, EMA, and other agencies have approved several biologic antibodies targeting the inflammatory cytokines IL-4, IL-13, IL-5, and others for asthma.
pharmaphorum
SEPTEMBER 21, 2022
The European Commission has followed the lead of the US FDA and approved AstraZeneca’s Tezspire as an add-on maintenance therapy for patients with severe asthma, becoming the first and only biologic that can be used in all patients, and not restricted to those with specific forms of the disease. billion or more.

European Pharmaceutical Review
AUGUST 8, 2023
Tom Keith-Roach, President of AstraZeneca UK announced: “We are pleased that the SMC has now accepted Forxiga (dapagliflozin) for use in patients with chronic heart failure with preserved or mildly reduced ejection fraction and Tezspire (tezepelumab) has been accepted for restricted use in severe asthma within NHS Scotland.”

Pharmacy Times
MARCH 15, 2024
The study showed no clinical differences for remission or asthma at age 28 years based on lung function, body mass index, daily smoking, exposure to parental tobacco smoke, or house dampness.
pharmaphorum
AUGUST 12, 2022
Patient recruitment specialist Clariness will deploy an app aimed at helping asthma patients monitor environment triggers and report symptoms in the clinical trials its support, thanks to a partnership with digital health firm DailyBreath.

Pharmaceutical Technology
JUNE 9, 2023
Clinical-stage biotech company Upstream Bio has raised $200m in a Series B financing round to advance its UPB-101 to treat allergic and inflammatory diseases. Upstream Bio will use the funds to advance its UPB-101 into Phase II trials to treat asthma and chronic rhinosinusitis with nasal polyps (CRSwNP).
pharmaphorum
JANUARY 12, 2023
Airsupra (PT027), a new asthma rescue treatment developed by AstraZeneca (AZ) and development partner Avillion, has been approved by the US Food and Drug Administration (FDA). An estimated 136 million asthma exacerbations occur globally each year, 10 million of those in the US.

European Pharmaceutical Review
NOVEMBER 21, 2023
If successful this could lead to regulatory submissions in 2025, supporting the health of asthma and COPD patients and making a significant positive impact on our transition to a more environmentally sustainable future,” GSK’s Chief Executive Officer, Emma Walmsley commented.

pharmaphorum
NOVEMBER 16, 2022
There’s plenty in the pipeline to entice a potential buyer, however, including several mid-stage clinical candidates, such as EPO inhibitor LKA651 for diabetic retinopathy, Factor B inhibitor iptacopan for AMD, and gene therapy PPY988 for geographic atrophy. Novartis has said it will not comment on market speculation.

Pharmaceutical Technology
NOVEMBER 18, 2023
Rilzabrutinib is under clinical development by Principia Biopharma and currently in Phase II for Asthma.
pharmaphorum
NOVEMBER 9, 2022
AstraZeneca has moved a step closer to bringing a first-in-class drug to market in the US that can be used to reduce severe asthma attacks, after an FDA advisory committee voted in favour of approval. The post FDA panel backs AZ’s PT027 asthma rescue drug, but in adults only appeared first on.

Pharmaceutical Technology
FEBRUARY 24, 2023
Amlitelimab is under clinical development by Kymab and currently in Phase II for Asthma. According to GlobalData, Phase II drugs for Asthma have a 27% phase transition success rate (PTSR) indication benchmark for progressing into Phase III. It is administered through intravenous and subcutaneous routes as an infusion.
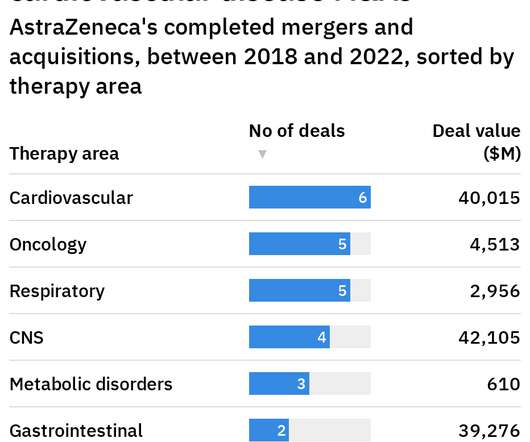
Pharmaceutical Technology
SEPTEMBER 5, 2022
An example of AstraZeneca’s deals in the cardiovascular disease clinical landscape is its agreement with US-based Dogma Therapeutics. The company currently has five Phase III clinical trials in progress for respiratory diseases that aim to target conditions such as asthma and COPD.

pharmaphorum
NOVEMBER 25, 2022
Case study: severe asthma. Some years ago, as new biologic treatments for severe, uncontrolled asthma were in development, and specific patient groups were being proposed for these treatments based upon blood eosinophil (EOS) counts. Therefore, start at the point the data allows – usually, prevalence per 100,000.

European Pharmaceutical Review
DECEMBER 20, 2023
FDA’s priory review was based on positive results from an ongoing Phase III trial, sponsored by the US National Institutes of Health (NIH)” FDA’s priory review was based on positive results from an ongoing Phase III clinical trial, OUtMATCH, sponsored by the US National Institutes of Health (NIH).

pharmaphorum
NOVEMBER 14, 2022
Dupixent was cleared by the US FDA for adults with PN in September, making it an option for an estimated 75,000 patients with the condition, and adding to the drug’s earlier approvals in atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic oesophagitis. Sales of Dupixent rocketed 45% to around $2.4
pharmaphorum
DECEMBER 19, 2022
It would provide an alternative to standard breathing exercises for patients with conditions that affect breathing, including long-term respiratory diseases like asthma, chronic obstructive pulmonary disorder (COPD) and cystic fibrosis, which can be resource-intensive as they require patients to be overseen by a healthcare worker.
Pharmaceutical Technology
APRIL 11, 2023
Under the FDA’s drug safety Sentinel Initiative, the partnership will develop AI tools to collect key information from clinical notes within electronic health records (EHR). During the two-year project, the companies will assess the mental health side effects of the asthma drug, montelukast.
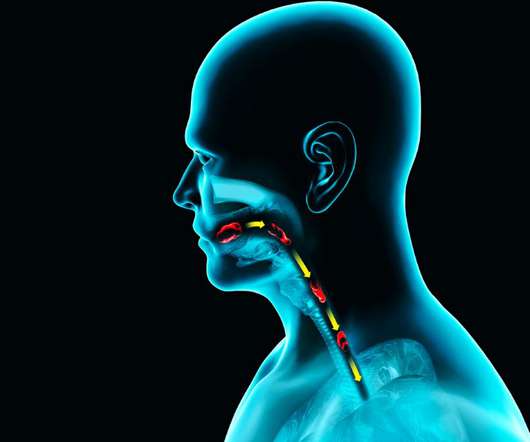
European Pharmaceutical Review
JANUARY 31, 2023
It is the “… first and only targeted treatment option clinically proven to reduce both oesophageal inflammation and damage,” explained Dr Naimish Patel, Head of Global Development, Immunology and Inflammation at Sanofi. More than 500,000 patients have been treated with Dupixent globally.

Clarivate
AUGUST 17, 2022
In our latest podcast, Ideas to Innovation, we speak with Dr. Grace Lomax, the clinical director at Patient Connect, part of Clarivate. Dr. Grace Lomax, who co-founded Patient Connect with her sister in 2008, came up with the idea for the solution when she started asking patients in her clinic what information they wanted when and how.

pharmaphorum
OCTOBER 26, 2022
Boehringer Ingelheim has moved one of its top pipeline prospects – oral phosphodiesterase 4B (PDE4B) inhibitor BI 1015550 – into late-stage clinical testing for idiopathic pulmonary fibrosis and other interstitial lung diseases (ILDs). ” The post Seeking Ofev successor, Boehringer takes PDE4B drug into phase 3 appeared first on.
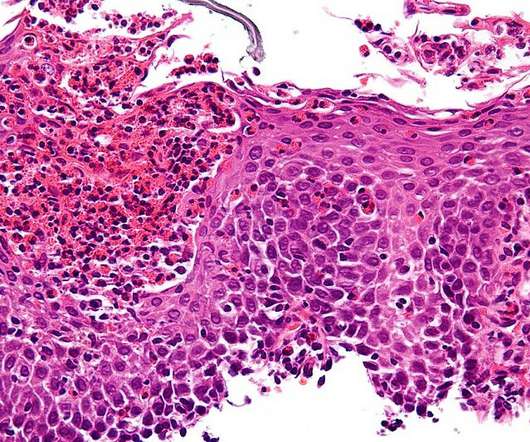
Pharmaceutical Technology
JANUARY 30, 2023
It has already been approved in many countries worldwide for patients in different age groups with CRSwNP, atopic dermatitis, asthma, EoE or prurigo nodularis. The fully human monoclonal antibody Dupixent has been designed to block the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways.
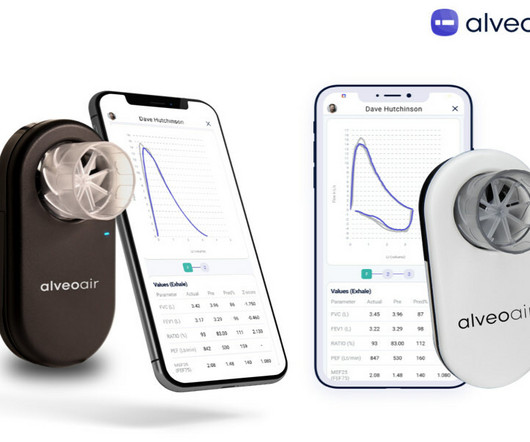
Legacy MEDSearch
SEPTEMBER 7, 2023
Anil Kukreja, VP of Medical Affairs and Regulatory at AstraZeneca Pharma India, praised the FDA clearance as a milestone, highlighting alveofit’s role in optimized diagnosis and management of lung disorders such as asthma and COPD. Earlier this year, to cater to the growing U.S. market, alveofit® inaugurated its office in New York.

pharmaphorum
SEPTEMBER 20, 2022
According to CytoReason its platform “turns biological disease models into actionable insights that enable biotech and pharma companies to shorten clinical trial phases, reduce drug development costs, identify new opportunities and increase the likelihood of FDA drug approval.”

Pharmaceutical Technology
JULY 18, 2022
These include the ongoing clinical development of AstraZeneca’s Fasenra (benralizumab), Insmed’s brensocatib and Novartis’ icenticaftor (QBW251). receptor antagonist indicated for the treatment of severe eosinophilic asthma, is currently being investigated in the Phase III MAHALE trial (NCT05006573).

Pharmaceutical Technology
DECEMBER 7, 2022
On 29 November, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) warned of some new and potentially serious eye-related side effects associated with Dupixent, an interleukin (IL)-4/13 inhibitor drug used in the treatment of numerous allergic indications such as atopic dermatitis, asthma and nasal polyps.

pharmaphorum
AUGUST 4, 2022
Amgen sells a biosimilar version of rituximab so Tavneos slots in alongside that therapy, and the company also gets three early clinical-stage pipeline candidates, namely an oral PD-1/PD-L1 inhibitor and two CC chemokine receptor-targeting drugs for inflammatory diseases.

Clarify Health
APRIL 1, 2021
Many healthcare and life sciences organizations recognize the impact of top social determinants of health on outcomes and cost, prompting them to invest in programs to address the non-clinical factors in patients’ lives. That is why Clarify Health has spent years creating the most SDoH-decorated dataset in the industry.
pharmaphorum
JULY 28, 2022
billion ($2 billion) – ahead of analyst expectations – driven by buoyant demand in its atopic dermatitis and severe asthma indications fuelled by approvals in earlier age groups. Sales of Dupixent (dupilumab) rocketed 43% to €1.96 The post Dupixent drives Sanofi to hike its full-year profit forecasts appeared first on.
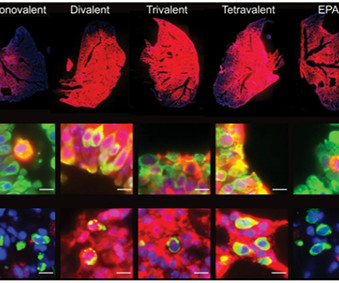
Medgadget
MAY 30, 2023
Researchers at UMass Chan Medical School have developed a small interfering RNA (siRNA) technology that is stable enough for inhalation into the lungs, where it can potentially treat diseases as diverse as asthma, pulmonary fibrosis, and viral infections such as COVID-19.

pharmaphorum
OCTOBER 27, 2022
The Swiss biotech is seeking a priority review for vamorolone, which was licensed from US biotech ReveraGen BioPharma in 2020 after Santhera its former DMD therapy candidate idebenone failed clinical testing and was abandoned. The post Santhera seeks speedy FDA review of Duchenne drug vamorolone appeared first on.

Legacy MEDSearch
JULY 18, 2023
“ReddyPort’s patented elbow is central to the eco-system we are building to help mitigate clinical obstacles tied to NIV therapy from dry-mouth, oral biofilm accumulation to speech recognition,” says Tony Lair, ReddyPort CEO. 7 In addition, NIV is utilized to wean patients off mechanical ventilation.

pharmaphorum
JANUARY 24, 2023
The partnership between Sanofi and CytoReason – for an undisclosed, multimillion dollar amount – forms part of an ongoing collaboration begun in 2021, which originally focused on obtaining advanced mechanistic insights for asthma endotypes. CytoReason develops computational disease models.

pharmaphorum
SEPTEMBER 15, 2022
The Clinical Data Interchange Standards Consortium (CDISC) is dedicated to the improvement of medical research through data standardization. This enables regulatory reviewers to evaluate and process clinical trials more effectively. In this blog, we provide an overview of the CDISC standards used in clinical research.
Pharmaceutical Technology
JULY 21, 2022
This includes 600,000 packs of antibiotics, painkillers, cardiovascular, and oncology treatments from Novartis, and antibiotics, asthma medication, painkillers, and essential childhood vaccines from GSK. Drug pricing and gouging.
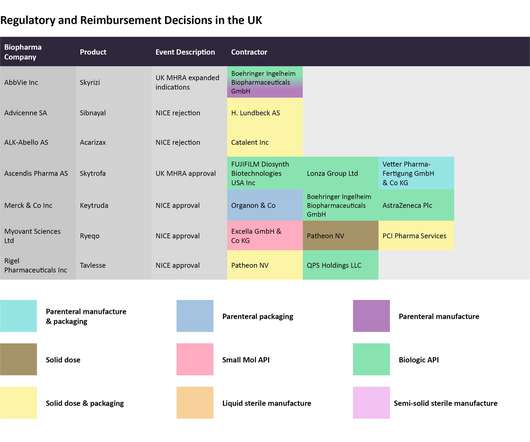
Pharmaceutical Technology
DECEMBER 12, 2022
The successful implementation of these contracts is necessary to ensure that a newly approved drug or an investigational therapy that is ready to advance to the next pivotal stage of clinical evaluation is manufactured in a timely manner. Regulatory decisions and clinical trial news.

pharmaphorum
JULY 25, 2022
Vabysmo still has a mountain to climb to catch Eylea, but Roche continues to build the clinical case for its drug reporting two-year results with the drug in wet AMD earlier this month which suggested it continued to be effective whilst cutting the number of injections into the eye needed by patients.

European Pharmaceutical Review
JULY 26, 2022
The CHMP gave a positive opinion for Tezspire (Tezepelumab), intended as an add-on treatment in adult and adolescent patients with severe asthma. Hybrid medicines rely in part on the results of pre-clinical tests and clinical trials of an already authorised reference product and in part on new data.

PM360
SEPTEMBER 21, 2023
COPD symptoms vary from patient to patient, Dr. Feigler says, and therefore doctors should continue to ask about each patient’s activities and lifestyle in addition to typical clinical measures to better understand if their COPD is progressing.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content