How health plans can leverage competitive intelligence data today to drive impact in 2024
Clarify Health
DECEMBER 6, 2023
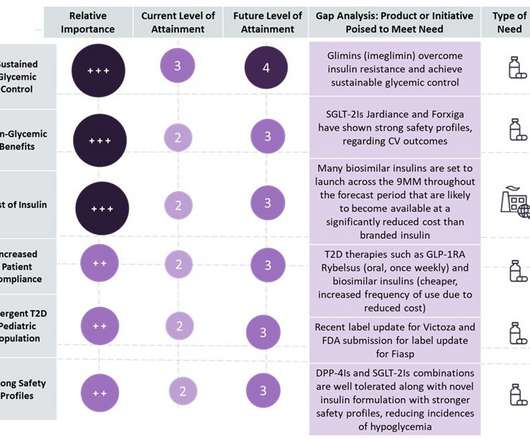
The strategic use of competitive intelligence data is increasingly becoming a crucial factor for healthcare payers looking to make informed decisions around strategic market expansion and strengthen their market position.












































Let's personalize your content