Pharmaceutical industry: 2023 in retrospect
European Pharmaceutical Review
JANUARY 29, 2024
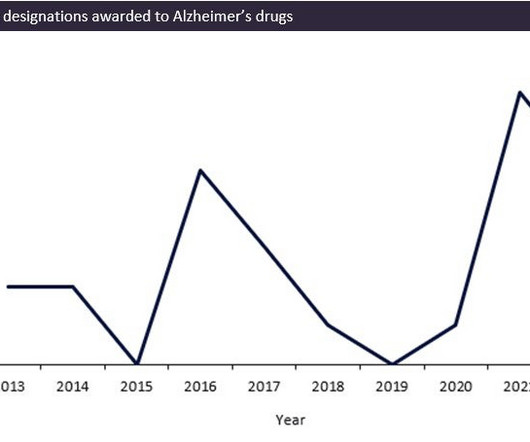
N-Nitrosamines By far the biggest issue bedevilling industry during 2023 was the continuing N-nitrosamine contamination saga, which was covered in the third issue of EPR 2023. During August 2023 the FDA also updated its guidance for NDSRIs 7 and it was closely aligned (but not identical) to EMA’s evolving guidance.






































Let's personalize your content