EC grants orphan drug designation for Precigen’s PRGN-2012
Pharmaceutical Technology
JANUARY 17, 2024
The EC has granted orphan drug designation (ODD) for Precigen's PRGN-2012 aimed at treating recurrent respiratory papillomatosis (RRP).
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
 2012 Related Topics
2012 Related Topics 
Pharmaceutical Technology
JANUARY 17, 2024
The EC has granted orphan drug designation (ODD) for Precigen's PRGN-2012 aimed at treating recurrent respiratory papillomatosis (RRP).

Fierce Pharma
JULY 8, 2024
The traditional approvals follow initial accelerated approvals granted by the FDA in 2012 and the European Commission in 2014. It took many years, but Johnson & Johnson’s tuberculosis med Sirturo can finally claim full approvals in the U.S. and Europe following initial conditional nods. |
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
JANUARY 27, 2023
Indeed, Initial Public Offerings (IPOs) raised just £28 million – the worst annual haul since 2012 – and, during the entirety of last year, UK biotech raised just £1.8 The post UK biotech sees worst funding downturn since 2012 appeared first on. billion, way down from £4.5 billion in 2021. billion raised.

Fierce Pharma
NOVEMBER 21, 2023
The lawsuit further accuses the companies of manipulating quality-control testing from 2012 to 2018 to ensure samples received passing grades. . | In its lawsuit, Texas says the companies knew that deficient manufacturing processes compromised the effectiveness of Quillivant XR.

Copyright Clearance Center
OCTOBER 24, 2023
Since 2012, the John Maddox Prize has recognized individuals who stand up for science and advance public discussions around challenging topics.

MedCity News
JULY 24, 2023
In 2020, 46.9% of all novel drugs approved by the FDA were for rare diseases, as compared to 23.5% Investment is expected to continue trending upward, with estimates suggesting that global spending on rare disease therapies will reach $260 billion by the end of 2025.

PharmaTech
JANUARY 11, 2024
Since 2012, we’ve been the industry leader in custom mRNA synthesis for research and clinical applications. Want to increase the probability success for your discovery mRNA program? Partner with TriLink BioTechnologies®. Read on to see how TriLink® is determined to deliver you success.

Legacy MEDSearch
MAY 3, 2023
Leadership includes Joy Celebre (recruiting since 1996, joined in 2008), Carolyn Jones (recruiting since 1995, joined in 2011), Arshad Alam (recruiting since 1995, joined 2017), John White (Talent Sourcing Manager, joined 2012), and Partner Chris Miclot, (joined 2012). “In

Clarivate
AUGUST 2, 2022
The following blog post summarizes key findings from the latest CIRS R&D Briefing, New drug approvals in six major authorities 2012-2021. The resulting analyses, published annually since 2012, give unique insights into regulatory processes and practices, identify where improvements can be made and inform company and agency strategies.

PM360
JUNE 11, 2024
His visionary work has set a high standard, and I am committed to upholding and advancing the spirit and excellence that Calcium was founded with in 2012. Together with our talented team, I look forward to building on this strong foundation and driving our creative vision to new heights.” “We
European Pharmaceutical Review
FEBRUARY 29, 2024
and Genmab A/S made an agreement in August 2012, in which Janssen would be granted an exclusive license to develop, manufacture and commercialise daratumumab. ” The Janssen Pharmaceutical Companies of Johnson & Johnson noted that Janssen Biotech, Inc.

Pharma Marketing Network
JULY 17, 2023
Anna was named a Top 100 PharmaVoice “most inspiring leader” in 2012. With Anna at the helm, MedEvoke has quickly become a leading partner to many top-20 pharmaceutical corporations.
European Pharmaceutical Review
APRIL 27, 2023
AAVs evolving the in vivo gene therapy space In 2012, the European Medicines Agency (EMA) approved the first AAV gene therapy, Glybera (Alipogene tiparvovec). Achieving this in the relatively new and dynamic CGT regulatory landscape will rely on flexible and agile developers and manufacturers.

Clarivate
OCTOBER 7, 2021
Every year since 2012, Clarivate has identified the Top 100 Global Innovators. In this episode of Ideas to Innovation , we welcome Bernhard Quendt of Thales, one of the world’s Top 100 Global Innovators. Listen to the podcast.
Pharmacy Times
AUGUST 17, 2022
Analysis looks at those with arthritis, chronic pain, headaches, lower back pain, and neuropathic pain between 2012 and 2019.

European Pharmaceutical Review
JULY 4, 2024
2 It was first approved as Fulyzaq in December 2012 and in October 2016, Napo Pharmaceuticals launched the new brand, Mytesi. 14 Marketing authorisation In the UK, medicinal products placed on the market are required to have marketing authorisations in accordance with The Human Medicines Regulations 2012 (S.I. 2012/1916).

European Pharmaceutical Review
APRIL 27, 2023
Growth has been rapid since Europe’s first gene therapy approval in 2012, 2 and the first US Food and Drug Administration (FDA) approval of a gene therapy in 2017. 3 Any sector would struggle to keep up with expansion of this speed, but the inherent difficulty of working with and producing biologics adds another layer of complexity.

European Pharmaceutical Review
FEBRUARY 27, 2024
In 2012, Dame June was elected as the first chair of the European Pharmacovigilance Risk Assessment Committee, MHRA added. Her educational background includes training in medicine in Oxford after completing a master’s degree by research in Pharmacology.

Pharmaceutical Representative Training
JUNE 22, 2024
Non-Compliance CASE 3 : Case Summary: In 2012, another company paid a record $3 billion to settle charges of illegal promotion of prescription drugs, failure to report safety data, and false price reporting. The settlement was the largest healthcare fraud settlement in U.S. link] Department of Justice. link] Reuters. link] The Guardian.
European Pharmaceutical Review
NOVEMBER 22, 2023
Since most patients have an initial response to the treatment, 80 percent will experience recurrence and require subsequent therapies, according to a 2012 paper published in Annals of Oncology.
pharmaphorum
NOVEMBER 11, 2022
Black/African American participation in trials has been declining over the past decade, says the report, falling from around 12% in 2012 to less than 10% in 2019 and 2020. For Hispanics, participation in trials increased from 7% in 2012 to a high of just under 10% in 2017 but fell back to levels of around 8% to 9% in the past two years.
World of DTC Marketing
NOVEMBER 24, 2021
million people diagnosed with cancer from 2000 to 2012 drained their life’s assets within two years. One-quarter of all cancer patients chose not to fill a prescription due to cost, according to a 2013 study in The Oncologist. According to a study published in the American Journal of Medicine, more than 42 percent of the 9.5

pharmaphorum
JULY 22, 2022
The CMA delivered a preliminary judgment in the case last year which concluded that Pfizer and Flynn abused a dominant position in phenytoin sodium capsules, causing NHS spending on the drug to balloon from around £2 million a year in 2012 to £50 million the following year.

pharmaphorum
JANUARY 3, 2023
In an illustrious career, he also served as president of the Royal Society of Medicine (RSM) from 2012 to 2014, as well as chairman of the Committee on Safety of Medicines (1992-1998), chairman of the Advisory Council on the Misuse of Drugs (1998-2008), and chairman of UK Biobank (2012-2019), and was also appointed honorary professor at the London (..)

European Pharmaceutical Review
DECEMBER 14, 2023
Between 1999 and 2001, it supported 17 percent of withdrawals, between 2002 and 2011, it supported 26 percent of withdrawals and by 2012 to 2016, 80 percent of withdrawals were supported by observational studies (cohort, case-control, or other epidemiologic design). Meta-analyses contributed least frequently (31.4 Lane S, Lynn E, Shakir S.
European Pharmaceutical Review
DECEMBER 21, 2022
The US Food and Drug Administration (FDA) created live biotherapeutic products (LBP) as a new category in the 2012 guidelines. 1 It defined LBPs as drug products containing live microorganism(s) to be used to prevent, treat or cure a disease or condition in human beings.

European Pharmaceutical Review
JANUARY 16, 2023
The Food and Drug Administration Safety and Innovation Act, enacted in 2012, permitted the FDA to enter into agreements to recognise drug inspections conducted by foreign regulatory authorities determined to be capable of conducting inspections that meet US requirements.
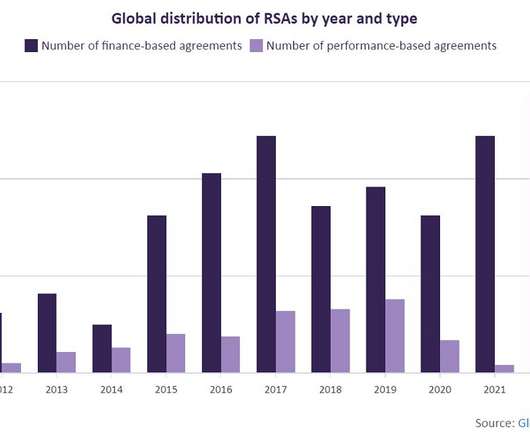
Pharmaceutical Technology
FEBRUARY 10, 2023
Risk-sharing agreements grow at a rate of 24% Since 2012, the increasing number of countries pursuing RSAs has been limited, but the elevated volume of agreements arranged each year has resulted in an average annual growth rate (AAGR) of 24%.

European Pharmaceutical Review
JUNE 7, 2023
Supporting the UK’s cell and gene therapy industry Since 2012, there has been over £5.5 Additionally, a team of technical and clinical adoption experts based in offices at the site will work closely with collaborators to help them bring cell therapies to clinical trials and the market.

Pharmacy Times
MAY 22, 2023
Blasini joined FCS in 2012 as a hematopathologist and has served as co-director of the Pathology Laboratory since 2018.

Pharmaceutical Technology
MAY 10, 2023
The FDA approved Truvada as the first PrEP treatment in July 2012, with Descovy receiving a nod for this use in October 2019. Gilead markets both Truvada and its successor Descovy. While both Descovy and Truvada contain emtricitabine, they feature different forms of the antiviral tenofovir.

European Pharmaceutical Review
JULY 20, 2022
Our research looked at products that were voluntarily withdrawn, or had their licence revoked or suspended within the EU for a safety reason between 2012 and 2016. The Human Medicines Regulations 2012, UK statutory instruments, 2012 No 1916, PART 5, Revocation, variation and suspension of marketing authorisation. Study focus.

World of DTC Marketing
MARCH 23, 2022
The program was codified into law under the Food and Drug Safety and Innovation Act (FDASIA) in 2012. Accelerated Approval was developed in 1992 in response to the HIV/AIDS crisis and has led to expedited drug and biologic approvals in several disease areas across the FDA.

Pharma Leaders
JUNE 19, 2023
Founded in 2012, Lucis develops and licenses new and exclusive medicines. The acquisition will also allow Rosemont to widen its portfolio and foray into the unit dose or sachet market via the pipeline products and expand its business via recent Lucis’ product launches in 2021/22.
European Pharmaceutical Review
FEBRUARY 10, 2023
Since its opening in 2012, occupier companies have secured £2.9bn in finance, making the cluster one of the most attractive for investment in biotechnology in the UK. It provides companies access to specialist equipment, mentoring and finance suited to their stage of development.

ProSellus
JULY 18, 2018
What’s more, since 2012 and 2016 (the time frame of […]. In fact, in a 2017 report for the nonprofit Physician Advocacy Institute, revealed that 5,000 physician practices and over 14,000 physicians have been ‘scooped up’ by hospitals between July 2015 and July 2016 alone. appeared first on ProSellus.
World of DTC Marketing
MARCH 27, 2022
The national rate of suicidal ideation among adults has increased every year since 2011-2012. Suicidal ideation continues to increase among adults in the U.S. 4.58% of adults report having serious thoughts of suicide, an increase of 664,000 people from last year’s dataset. who are going untreated.
Pharmaceutical Technology
MAY 26, 2023
While the market was worth over $6 billion in 2012, this has now grown to nearly $48 billion only a decade later, said Avigayil Chalk, PhD, GlobalData’s Senior Oncology and Haematology analyst, at an immuno-oncology webinar held on May 23.

Impetus Digital
JANUARY 30, 2023
During his medical training at the University of Toronto, he co-led a research project at the University Health Network on hospital readmissions that inspired him to develop technology to help providers engage and monitor patients to prevent adverse outcomes, ultimately leading to him co-founding SeamlessMD in 2012.

PM360
MAY 21, 2024
Minding the Gap In 2012, I was invited to a life science and pharma event in London as part of the Olympics. I was lucky enough to enter academia with a number of women colleagues. It was only later in my career that I started noticing the gender gap in the pharma industry.

pharmaphorum
OCTOBER 26, 2022
The two drugmakers were fined in July, after the CMA upheld a preliminary judgment that Pfizer and Flynn had abused a dominant position in phenytoin sodium capsules, causing NHS spending on the drug to swell from around £2 million a year in 2012 to £50 million the following year.

European Pharmaceutical Review
MARCH 16, 2023
Ivacaftor – a breakthrough treatment The approval of IVA in 2012 marked a breakthrough in treating the disease. This is important for those with F508del, as it can increase the quantity of protein at the cell surface, where it can now be acted upon by IVA.

Pharmaceutical Technology
JUNE 30, 2022
In 2012, the company was acquired by Amgen and renamed Gensenta in 2020. Amgen Turkey noted that the company will maintain its operations and serve its patients in the region. Established as a laboratory in 1923, Gensenta became Mustafa Nevzat Pharmaceuticals in 1957.

pharmaphorum
SEPTEMBER 26, 2022
Other drugs used to treat ATL include Kyowa Kirin’s amti-CCR4 antibody Poteligeo (mogamulizumab), cleared by the MHLW for CCR4-positive ATL in 2012, as well as Bristol-Myers Squibb/Celgene’s oral therapy Revlimid (lenalidomide), which got a green light there five years ago.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content