The role of real-world evidence in the lifecycle of diagnostic, medical device, and digital therapeutic products
Clarify Health
JANUARY 12, 2022
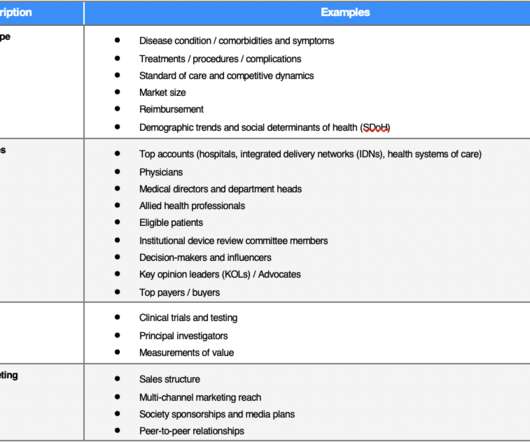
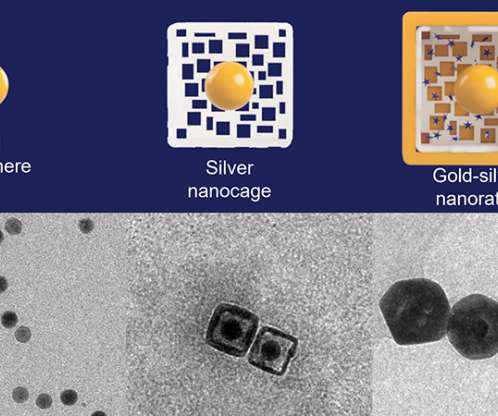
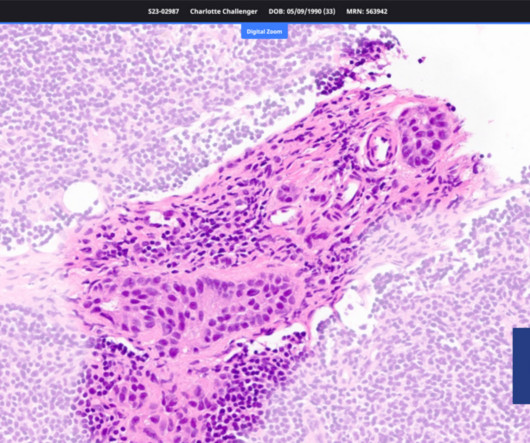
From FDA regulatory approvals to commercialization strategies to value demonstrations, real-world evidence (RWE) plays a pivotal role in the product lifecycle of diagnostics, medical devices, and digital therapeutics (DTx).








































Let's personalize your content