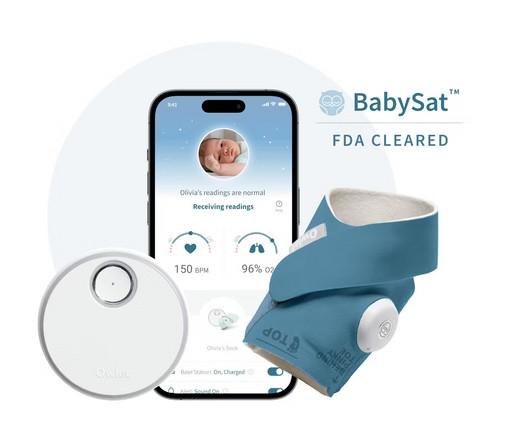
Owlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
Legacy MEDSearch
JUNE 20, 2023
Food and Drug Administration (“FDA”) of BabySat , the first medical pulse-oximetry device featuring its advanced, wire-free sock design. BabySat is a step forward in solving some of these exact challenges by bringing real-time medical grade infant monitoring into the home, while under the supervision of a physician. Are you hiring?































Let's personalize your content