With new FDA approval, Merck's Welireg takes another step toward blockbuster goal
Fierce Pharma
DECEMBER 15, 2023
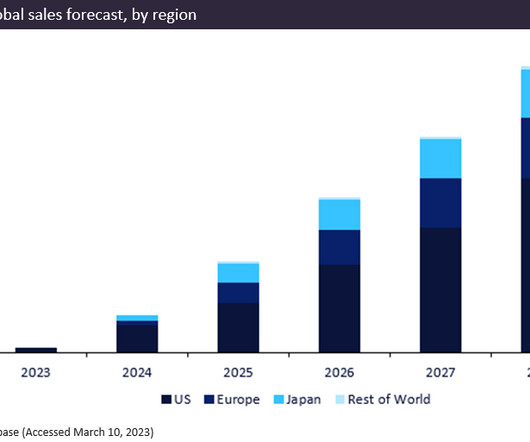
Since Merck secured approval for kidney cancer pill and blockbuster hopeful Welireg (belzutifan) in August of 2021, sales have grown slowly but surely. Since Merck secured approval for kidney cancer pill and blockbuster hopeful Welireg (belzutifan) in August of 2021, sales have grown slowly but surely.




































Let's personalize your content