Re-engineering proteins to develop novel immunotherapies
European Pharmaceutical Review
JULY 13, 2023
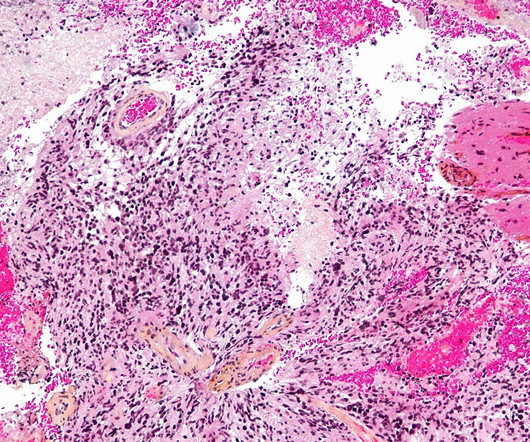
However, even as science advances, there remain patients who still need new treatment options. By re-engineering existing approaches that have already shown clinical promise, there is potential to bring further benefit to the patient community in its fight against cancer.



































Let's personalize your content