Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease
Fierce Pharma
MAY 19, 2023
Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease zbecker Fri, 05/19/2023 - 16:25

Fierce Pharma
MAY 19, 2023
Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease zbecker Fri, 05/19/2023 - 16:25

MedCity News
MAY 14, 2023
There is a huge opportunity for PBMs and health systems to use AI to help address the challenges surrounding the affordability and accessibility of medicines, from navigating formularies to resolving prior authorization. More automation in pharmacies and with ordering workflows is one piece of that puzzle.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Pharmaceutical Technology
MAY 19, 2023
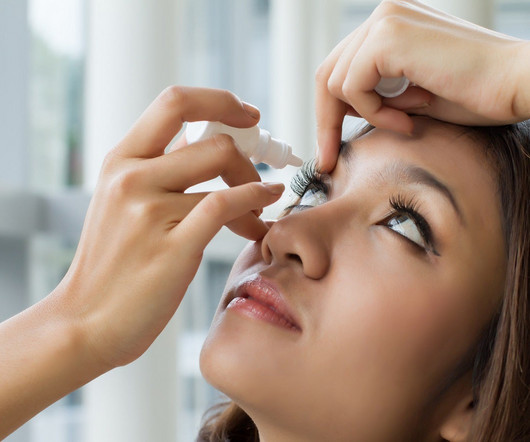
The US Food and Drug Administration (FDA) has granted approval to Bausch + Lomb and Novaliq’s Miebo (perfluorohexyloctane ophthalmic solution) to treat the signs and symptoms of dry eye disease (DED). Formerly known as NOV03, Miebo is a first-in-class eye drop designed for preventing the evaporation of excessive tears and restoring tear balance in evaporative DED patients.

Clarivate
MAY 18, 2023
As part of our on-going commitment to research integrity, we recently shared that we planned to investigate how we can provide greater transparency regarding which journals are covered in the Web of Science Core Collection. We thank our many community partners for their feedback and acknowledge the request for even more clarity around which journals are added or removed.

Fierce Pharma
MAY 17, 2023
Regulatory tracker: Another China-made PD-1 starts FDA journey as Elevar, Hengrui target big cancer type aliu Wed, 05/17/2023 - 09:41

MedCity News
MAY 15, 2023
Patient demand for digital payment communication — both reminders about bills, as well as text messages that enable bill payment — is on the rise, according to a recent report. This is in line with growing consumerism trends in the U.S. — patients want their healthcare payment experience to mimic the convenience and ease they have when they pay for things like travel or retail goods.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition. Whilst there is a range of FDA-approved biologics for Crohn’s disease, Rinvoq is the first approved oral product for the moderate to severe type of the disease.

Fierce Pharma
MAY 19, 2023
Intercept's NASH dreams may be dashed after FDA panel votes against Ocaliva's approval bid fkansteiner Fri, 05/19/2023 - 17:30

MedCity News
MAY 16, 2023
Hippocratic AI emerged from stealth, as well as announced $50 million in seed funding through a round co-led by General Catalyst and Andreessen Horowitz. The Palo Alto-based startup bills itself as the first large language model designed specifically for healthcare.
European Pharmaceutical Review
MAY 19, 2023
Final draft guidance has been published for the first National Institute for Health and Care Excellence (NICE)-recommended treatment for symptomatic chronic heart failure with preserved or mildly reduced ejection fraction. The regulatory body’s decision means up to 150,000 patients would be eligible for AstraZeneca-made dapagliflozin (Forxiga).

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

PM360
MAY 15, 2023
Maya Angelou said, “If you’re going to live, leave a legacy. Make a mark on the world that can’t be erased.” For nine years, we have looked to recognize those people in healthcare and life sciences who are making a mark on our industry, on patients, on colleagues, on the world. These people represent our ELITE 100: the individuals and teams who throughout their careers are building legacies marked by helping people live better—and hopefully longer—lives.

Fierce Pharma
MAY 19, 2023
AbbVie, Genmab's lymphoma drug Epkinly snags FDA approval in heated blood cancer race aliu Fri, 05/19/2023 - 11:21

MedCity News
MAY 19, 2023
Included Health and DispatchHealth are partnering to combine Included’s virtual care services with DispatchHealth’s home care services. If an Included physician notices a patient requires in-person support, the physician will send DispatchHealth to the patient’s home.
European Pharmaceutical Review
MAY 16, 2023
So far, 2023 has seen several major developments in stem cell-based therapies. Stem cells have the ability to self-renew. Their potential to differentiate into cells of different tissues makes them particularly interesting for applications in regenerative medicine. 1 Since the first therapy using stem cells was developed in 1957, only few stem cell-based therapies have entered the clinic 1 as advanced therapy medicinal products (ATMPs).

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

Pharmaceutical Technology
MAY 18, 2023
The European Medicines Agency (EMA) has published recommendations to increase communication and planning efforts in a bid stop the current medicine shortages becoming even worse. Within the 14-page document , published by an EMA special task force, are 10 recommendations of best practices that “marketing authorisation holders, wholesalers, distributors, and manufacturers can consider adopting to ensure continuity of medicinal product supply and reduce the impact of shortages”.

Fierce Pharma
MAY 19, 2023
Bausch + Lomb bags FDA approval for dry eye disease treatment Miebo zbecker Fri, 05/19/2023 - 09:39

MedCity News
MAY 14, 2023
We must engage, train and support more physicians as investigators to enable the opportunity for their patients to have access to clinical trials. As an industry, we should be adding anywhere from 10% to 20% new investigators every year to meet the patient access needs for clinical trials.
European Pharmaceutical Review
MAY 18, 2023
A new document published by European Medicines Agency (EMA) makes ten recommendations and outlines good practices “to ensure continuity in the supply of human medicines, prevent shortages and reduce their impact.” Medicine shortages are a global health problem and are increasingly affecting European countries, according to EMA. Shortages can lead to medicine rationing, delay in critical treatments and can mean patients may need to use less-effective alternatives and face an increased risk of me

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Pharmaceutical Technology
MAY 19, 2023
Myeloid Therapeutics has raised $73m to support the continued clinical development of its lead cell therapy programme, MT-101, in Phase I/II trials for T cell lymphoma. Led by Hatteras Investment Partners, the financing round has seen participation from existing investors, including 8VC, Alexandria Venture Investments and Newpath Partners, along with new investors Moore Strategic Ventures and ARCH Venture Partners.

Fierce Pharma
MAY 18, 2023
SCOTUS hands win to Sanofi, Regeneron in long-running PCSK9 feud with Amgen fkansteiner Thu, 05/18/2023 - 15:17

MedCity News
MAY 17, 2023
Five companies will take part in the showcase with technologies addressing mental health, care coordination, aging in place, physical therapy and more. Register for INVEST, scheduled for May 22-24, at the Ritz Carlton in Chicago!
European Pharmaceutical Review
MAY 18, 2023
Hepcludex ® (bulevirtide) is the first medicine to be conditionally licensed for chronic hepatitis delta virus (HDV) infection in Great Britain” The National Institute for Health and Care Excellence (NICE) has recommended NHS use of Hepcludex ® (bulevirtide). It is the first medicine to be conditionally licensed for chronic hepatitis delta virus (HDV) infection in Great Britain.
Pharmaceutical Technology
MAY 19, 2023
Multiple sclerosis (MS) is a primary autoimmune disease in which inflammation is a core contributor to the degeneration of the central nervous system (CNS), leading to neurological disability and affecting sensory, visual, motor, and autonomic systems. While MS is not a terminal diagnosis, the effect of the disease on the CNS can significantly impact patients’ independence and disturb their daily lives.

Fierce Pharma
MAY 16, 2023
FTC seeks to block Amgen's $28B Horizon buy in 'broadly negative' move for biopharma M&A: report aliu Tue, 05/16/2023 - 09:41

MedCity News
MAY 18, 2023
FDA approval of blockbuster AbbVie drug Rinvoq makes it the first oral therapy for moderately to severely active Crohn’s disease. The regulatory nod is the seventh for the drug, which belongs to a class of therapies called JAK inhibitors.

Copyright Clearance Center
MAY 16, 2023
May 16, 2023 – Danvers, Mass. – CCC , a leader in advancing copyright, accelerating knowledge, and powering innovation, was named a Bronze Stevie® Award winner for Customer Service Department of the Year in the 21 st annual American Business Awards ®. The American Business Awards are the premier business awards program in the U.S. Nicknamed the “Stevies” for the Greek word meaning “crowned,” the competition receives over 12,000 entries each year from organizations in 70 countries.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Pharmatutor
MAY 16, 2023
Pradhan Mantri Bhartiya Janaushadhi Pariyojana (PMBJP) : Making Quality Medicines Affordable and Accessible in India admin Tue, 05/16/2023 - 15:25 About Author Ramraj Choudhary M.Pharma, (Pharmaceutics) Dr. H.S Gour University, Sagar MP Current Position : Sr.

Fierce Pharma
MAY 16, 2023
AstraZeneca follows AbbVie, Teva in surprising departure from lobbying group PhRMA zbecker Tue, 05/16/2023 - 11:06

MedCity News
MAY 19, 2023
Bausch + Lomb drug Miebo is now FDA approved as a new treatment for dry eye disease. Unlike many products that rewet the eye, Miebo is designed to address one of the factors that leads to dry eyes.

Clarivate
MAY 18, 2023
Marko Damjanovic, Head of Corporate Sustainability at Clarivate, reviews our 2022 ESG milestones and highlights our 2023 goals for advancing our strategic commitments to the United Nations SDGs – all by accelerating our actions and by helping our customers achieve their goals. At Clarivate we put sustainability at the heart of our business goals and we want environment, social and governance issues to be the core of our global culture, serving as guiding principles for every decision we make.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content