Pfizer, Sanofi settle first California Zantac case slated for trial: report
Fierce Pharma
DECEMBER 22, 2022
Pfizer, Sanofi settle first California Zantac case slated for trial: report. zbecker. Thu, 12/22/2022 - 12:38.

Fierce Pharma
DECEMBER 22, 2022
Pfizer, Sanofi settle first California Zantac case slated for trial: report. zbecker. Thu, 12/22/2022 - 12:38.

European Pharmaceutical Review
DECEMBER 22, 2022
Moderna, Inc. has finalised a strategic partnership with the UK government to establish a state-of-the-art mRNA vaccine research, development, and manufacturing facility in the UK. This milestone follows the agreement in principle between Moderna and the UK Government, announced in June 2022. The Moderna Innovation and Technology Centre (MITC) is intended to provide access to a UK-made supply of COVID-19 jabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
DECEMBER 22, 2022
The ability to adapt has proven paramount over the past few years and it is fundamentally reshaping healthcare IT. In 2023, that shape will increasingly form around the cloud.

pharmaphorum
DECEMBER 22, 2022
Construction will start early next year of a new manufacturing centre in the UK with the capacity to produce 250 million vaccine doses per year, the centrepiece of a 10-year alliance between the government and US biotech Moderna. The government said today it has finalised the partnership – agreed in principle earlier this year and estimated to be worth in the region of $1.2 billion – although it is not revealing the financial details, as these are “commercially sensitive.” The overar

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

MedCity News
DECEMBER 22, 2022
Engineers see everything as a system, know how to design under constraints, and recognize the need for trade-offs. Adopting an engineering mindset in oncology research can fix all the broken constituent processes like patient enrollment to systematize clinical trials.
European Pharmaceutical Review
DECEMBER 22, 2022
The success of mRNA vaccines against SARS-CoV-2 has quickly catapulted mRNA therapeutics as a disruptive, expanding drug category” The term ‘mRNA’ has become commonplace globally. mRNA technologies have emerged as an innovative and effective approach to developing new drugs that can potentially transform existing therapies or target difficult‑to‑treat indications including respiratory, cardiac, metabolic and autoimmune diseases, as well as cancer.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
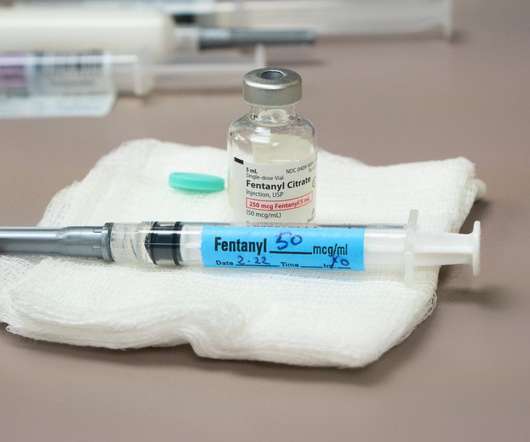
Pharmaceutical Technology
DECEMBER 22, 2022
Fentanyl is a powerful opioid (a narcotic analgesic that is at least partly synthetic) that is being trafficked in increasing quantities from Mexico to the US by cartels. The Drug Enforcement Administration (DEA) recently announced that it had seized 10,000 pounds of illicit fentanyl in 2022 and pointed out that this volume represented enough fentanyl to kill every American.

MedCity News
DECEMBER 22, 2022
The first regulatory approval of an allogeneic cell therapy goes to Atara Biotherapeutics. Other regulatory news highlights from the past week include proposed reforms of the FDA’s accelerated approval process, approval of the first gene therapy for bladder cancer, and a clinical hold for a biotech developing a new class of medicines.

PharmaVoice
DECEMBER 22, 2022
Art and science are often viewed as diametric opposites, but these industry insiders say their passion for painting blends into their pharma work.

MedCity News
DECEMBER 22, 2022
The future towards the safer treatment of pain will come as biopharma, and investor communities appreciate the significant opportunities in supporting scientifically rigorous and clinically validated lead assets for the safer treatment of pain.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

PharmExec
DECEMBER 22, 2022
Michael Ku, VP of global clinical supply at Pfizer, shares insights for the c-suite as well as reveals behind-the-scenes insight into Pfizer’s ability to deliver a COVID-19 vaccine swiftly.

MedCity News
DECEMBER 22, 2022
Hospitals’ operational margins have shrunk dramatically this year. Now more than ever, hospitals need to ensure they’re getting the most out of their investments in technology. Clear governance structures, effective communication and detailed training are among the factors hospitals need in order to get a significant return on investment for technology deployments, according to a new report.

pharmaphorum
DECEMBER 22, 2022
Four Japanese drugmakers – Astellas, Eisai, Daiichi Sankyo, and Takeda – have joined forces in a partnership intended to reduce the environmental impact of pharmaceutical packaging. The companies say they will promote the use of greener packaging for their products by sharing knowledge on technologies that can reduce their environmental impact , including blister packs made of biomass- rather than petroleum-derived plastics and more compact packaging, as well as recycled and recyclable materials

MedCity News
DECEMBER 22, 2022
Never has there been a time in healthcare when listening and understanding have been more critical. Issues of today are divisive, complex, and layered without easy solutions. We cannot possibly begin to fix without slowing down to listen through every channel available.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
European Pharmaceutical Review
DECEMBER 22, 2022
Gilead-owned Kite has agreed to acquire Tmunity Therapeutics, a clinical-stage biotech focused on next-generation CAR T-therapies. The value of the transaction was not disclosed. The acquisition of Tmunity complements Kite’s existing in-house cell therapy research capabilities by adding additional pipeline assets, platform capabilities, and a strategic research and licensing agreement with the University of Pennsylvania, Specifically, it will provide Kite with rapid manufacturing processes , as

MedCity News
DECEMBER 22, 2022
Blue Cross and Blue Shield of Minnesota formed a value-based arrangement with Minnesota Oncology in 2019. Preliminary data show that it led to a reduction in the total cost of care, emergency visits and inpatient admissions.
pharmaphorum
DECEMBER 22, 2022
To conclude pharmaphorum’s look back at day one of The Economist’s 8th Annual World Cancer Series congress in Brussels, Belgium, in November – where the foci were “innovation, equity, and excellence” – after the panel, ‘The future of European cancer control in a time of crisis’, came The Economist’s health policy editor Natasha Loder’s interview with Dame Kate Bingham, managing partner at SV Health Investors and former chair of the UK Government’s Vaccine Taskforce during the pandemic.
PharmaVoice
DECEMBER 22, 2022
New Alzheimer's drugs are poised to change the market — and in preclinical arenas, researchers are learning more and more about the disease.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

pharmaphorum
DECEMBER 22, 2022
Following an invite to fly into Parma, Italy to witness first-hand the press launch of the considerable structure that is the Chiesi Group’s new €85 million Biotech Centre of Excellence, pharmaphorum web editor Nicole Raleigh spoke with Chiesi’s head of global manufacturing, Antonio Magnelli. In brief, the European plan for Chiesi is in-house drug development of biologicals and rare disease targeting.

MedCity News
DECEMBER 22, 2022
AI chatbots and automated responses are great tools for service providers to leverage when it […].

pharmaphorum
DECEMBER 22, 2022
The life sciences industry is flexing towards innovation in new areas, faster than ever before, and increasing patient care in astonishing ways. Decentralised oncology trials, for example, have shown actual predictive outcome value. We can now measure patient activity, steps, and movement continuously and in real time, which serves as a new potential indicator of treatment effectiveness. 1 And, digital therapeutics have emerged as an effective treatment for chronic diseases, including mental ill

PharmaTimes
DECEMBER 22, 2022
Vital financing will help boost diagnosis, treatment and prospects of patient groups

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
pharmaphorum
DECEMBER 22, 2022
One of the most highly-anticipated developments in pharma next year will be the FDA’s verdict on Eisai’s accelerated application for lecanemab in Alzheimer’s disease, due early in January. The amyloid beta-targeting antibody has been filed on the strength of the phase 2b Study 201, with supporting data from the ongoing phase 3 CLARITY AD trial that, according to draft guidance (PDF) from the Institute for Clinical and Economic Review (ICER) in the US, is “promising but in

PharmaTimes
DECEMBER 22, 2022
Study uses carious drug combinations to increase survival among lung cancer patients

pharmaphorum
DECEMBER 22, 2022
AstraZeneca’s (AZ) Imfinzi (durvalumab) has been approved in the European Union (EU) as first-line treatment of adult patients with unresectable or metastatic biliary tract cancer (BTC) in combination with chemotherapy (gemcitabine plus cisplatin). BTC is a group of rare and aggressive cancers that occur in the bile ducts (cholangiocarcinoma) and gall bladder or ampulla of Vater.
Spotio
DECEMBER 22, 2022
We won’t beat around the bush: B2B sales is complex. To succeed in this space, you need to work hard, experiment with new strategies, and develop a winning B2B sales process that your reps can easily implement. That’s where we come in! Keep reading to learn the main differences between B2B sales and B2C sales, the foundations of the modern B2B selling process, and how to create a B2B sales model for your company that will reliably generate leads and revenue.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

PharmExec
DECEMBER 22, 2022
Angie Lindsey, vice president of marketing, Fresenius Kabi USA shares 2023 strategies for talent management strategies in life sciences, specifically as it relates to sales and marketing.

Legacy MEDSearch
DECEMBER 22, 2022
Endoluxe congratulates Keith Matheny, MD, FARS, of Collin County Ear Nose & Throat in Frisco, TX, for being the first ear, nose and throat (ENT) surgeon in the U.S. to use the Endoluxe Vision System for endoscopic sinus surgery. Dr. Matheny is a nationally-known innovator in the field of Otolaryngology and has developed or been involved with several game-changing technologies around the ENT space over the past two decades.

PharmExec
DECEMBER 22, 2022
Pratap Khedkar, CEO of ZS, a management consulting and technology firm focused on transforming global healthcare, shares his perspective on what 2023 holds for the sales and marketing vertical.

Pharmaceutical Technology
DECEMBER 22, 2022
Below you can find all the winners from the 2022 Excellence Awards which celebrate the greatest achievements and innovations in the industry. The programme provides a platform to recognise the people and companies that are driving change. Our programme is designed to highlight excellence within the sector by looking at a range of corporate activities including deals, business projects and company initiatives, both internal and in the community.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content