AbbVie's Skyrizi aces ulcerative colitis study en route to new IBD showdown with J&J, Takeda
Fierce Pharma
MARCH 23, 2023
AbbVie's Skyrizi aces ulcerative colitis study en route to new IBD showdown with J&J, Takeda aliu Thu, 03/23/2023 - 10:23

Fierce Pharma
MARCH 23, 2023
AbbVie's Skyrizi aces ulcerative colitis study en route to new IBD showdown with J&J, Takeda aliu Thu, 03/23/2023 - 10:23

MedCity News
MARCH 23, 2023
GrayMatters Health recently received 510(k) clearance from the FDA to market its flagship product, which is a self-neuromodulation digital therapy for PTSD. The device uses neurofeedback on specific biomarkers to help patients identify and utilize their own mental strategy for lowering their emotional response.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Fierce Pharma
MARCH 23, 2023
Fierce Pharma Asia—Takeda's $4B psoriasis data; Biohaven's neurology bet; Pfizer and Astellas' Xtandi wins aliu Thu, 03/23/2023 - 18:29

MedCity News
MARCH 23, 2023
Holon Solutions CEO Jon Zimmerman thinks more payers should invest in new technology to improve workloads for healthcare workers. The burnout crisis is too big for providers to solve by themselves, and many of the economic incentives for driving quality and efficiency in healthcare are housed within health plans and accountable care organizations, he said.

Fierce Pharma
MARCH 23, 2023
England's NICE signs off on PTC Therapeutics' $3.

MedCity News
MARCH 23, 2023
Conducted by researchers at the University of Colorado medical school, the new report is the latest to gauge the extent of a costly problem that federal regulators are working to solve: Information about doctors varies from provider directory to provider directory.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Pharmaceutical Technology
MARCH 23, 2023
Biohaven has purchased the exclusive global rights for oral, brain-penetrant dual Tyrosine Kinase 2 (TYK2)/Janus Kinase 1 (JAK1) inhibitor, BHV-8000 (previously TLL-041), which treats immune-mediated brain disorders , from Hangzhou Highlightll Pharmaceutical. The deal gives Biohaven global rights for the development of BHV-8000, excluding regions of China.

MedCity News
MARCH 23, 2023
Of the more than 15% of American adults who have past-due medical debt, about 73% owe some or all of the money to hospitals, according to a recent Urban Institute report. The American Hospital Association said short-term limited duration health plans and high-deductible health plans are to blame.
European Pharmaceutical Review
MARCH 23, 2023
Key data from the BOREAS Phase III trial, the first out of two Phase III trials in the Dupixent ® (dupilumab) development programme, has shown that o f 939 adult patients, 30 percent experienced reduction in moderate or severe acute chronic obstructive pulmonary disease (COPD) exacerbations over 52 weeks. The fully human monoclonal antibody is the first and only biologic to demonstrate significant improvements in lung function.
Medgadget
MARCH 23, 2023
Researchers at Eindhoven University of Technology in the Netherlands have developed a sensitive diagnostic test for viral pathogens that is suitable for use in low-resource regions. The test is based on CRISPR proteins that can detect viral genetic material but also incorporates luciferase proteins, which are bioluminescent proteins that are naturally found in fireflies and other creatures.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
MARCH 23, 2023
At the AHIP Medicare, Medicaid, Duals and Commercial Markets Forum held last week, two healthcare leaders from the Centers for Medicare and Medicaid Services (CMS) and CVS Health stressed that reducing maternal health disparities is a top priority, with several strategies at play. For CMS, the focus is on encouraging states to extend Medicaid postpartum coverage, while CVS Health is working to leverage doulas in their care delivery.
European Pharmaceutical Review
MARCH 23, 2023
Through its investment of approximately £600 million, Takeda plans to build a new manufacturing facility for plasma-derived therapies (PDTs) in Osaka, Japan. This is Takeda’s largest ever investment in manufacturing capacity expansion and will be the largest facility of its kind in Japan. This is Takeda’s largest ever investment in manufacturing capacity expansion and will be the largest facility of its kind in Japan.” “The new facility will provide a plasma fractionation capacity of more

MedCity News
MARCH 23, 2023
Alzheimer’s disease research at MIT spawned Cognito Therapeutics, a startup whose medical device uses light and sound to modulate electrical activity that supports brain health. The company’s Series B financing will support a Phase 3 test of its technology in patients with mild-to-moderate disease.
Copyright Clearance Center
MARCH 23, 2023
The post U.S. Copyright Office Launches AI Initiative, Including New Registration Guidance appeared first on Copyright Clearance Center.

Modus
MARCH 23, 2023
B2B selling has drastically shifted over the past few years. Remote working environments, buyer-driven sales processes, omnichannel selling - the list goes on. And sales and marketing alignment becomes more important than ever. Given the many changes, it’s important to hone in on some of the key traits of successful sellers.

PharmaTimes
MARCH 23, 2023
Eladocagene exuparvovec is for children with ultra-rare genetic disorder in final draft guidance
Pharmaceutical Technology
MARCH 23, 2023
Pfizer recently announced an agreement to acquire Seagen, a biotech company based in the US with four marketed oncology therapeutic agents and a rich pipeline. The deal, expected to be completed by the end of 2023, will see Pfizer pay $229 per Seagen share in cash for a total of $43bn, the largest deal for the sector in the past three years. Seagen specialises in developing antibody-drug conjugates (ADCs) which will complement Pfizer’s oncology portfolio.

PharmaTimes
MARCH 23, 2023
The partnership will involve progressing macrophage-targeting antibody treatment for cancer

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Pharmaceutical Technology
MARCH 23, 2023
This week, the Medicines and Healthcare products Regulatory Agency (MHRA) is introducing major changes to clinical trial regulation in the UK with expedited timelines at several stages. The MHRA hopes this new framework “will remove obstacles to innovation” and “streamline the regulation of clinical trials” amongst other things. These changes come following a public consultation with the Healthcare Research Authority, to which a government response was published on March 21.

Scott’s Directories
MARCH 23, 2023
Lead generation has created quite a buzz amongst marketers and business owners. As popular as it might be getting, its efficiency still depends on coming up with a robust, comprehensive strategy. How is a lead generation strategy created? How can lead generation companies help transform the way businesses sell their products and services, especially healthcare lead generation companies ?
Pharmaceutical Technology
MARCH 23, 2023
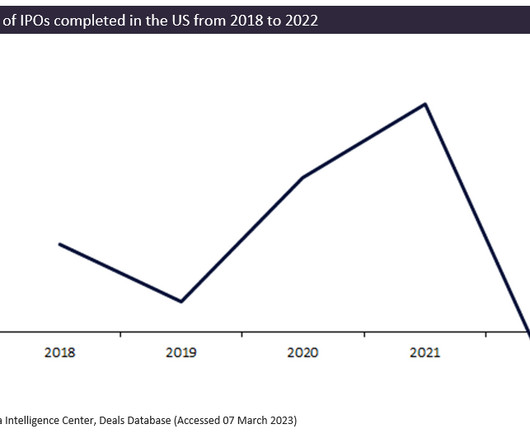
The biotech initial public offering (IPO) market saw a 79% decline in the number of completed IPOs from 2021 to 2022 in the US; however, this comes after the IPO boom in 2020 and 2021, according to GlobalData’s Pharma Intelligence Center Deals Database. An initial public offering is a process in which a private company sells its shares on the stock exchange to become public.

PharmaTech
MARCH 23, 2023
Wurster Fluid Bed Coating is one of the most utilized processes for microencapsulation of fine particles in pharmaceutical, dietary supplement, food, and other industries. Jared McDonald walks through a brief history of the invention of the process, what it is, and how it can be used to address specific needs for a product. A discussion of the importance of choosing an experienced CDMO follows.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharma Marketing Network
MARCH 23, 2023
Healthcare conferences have undergone significant changes since 2020 due to the COVID-19 pandemic. Prior to the pandemic, healthcare conferences were typically large in-person events, attracting thousands of attendees from around the world. However, with the outbreak of the virus and the need for social distancing, traditional in-person events have been disrupted, forcing the healthcare industry to find new ways to connect, share information, and collaborate.

PharmaVoice
MARCH 23, 2023
The clinical data space is rapidly changing the face of the industry, GSK's Mayank Anand said — and the way organizations adopt technology is a critical part of the equation.

Pharma Marketing Network
MARCH 23, 2023
Pharmaceutical companies have made major strides in developing new treatments for Alzheimer’s disease, offering hope for the millions of people affected by this debilitating condition. Alzheimer’s is a progressive brain disorder that destroys memory and cognitive abilities, and currently there is no cure. However, recent advancements in research have resulted in new drugs that show promising results in slowing the progression of the disease.
Progressive Medical
MARCH 23, 2023
Progressive Medical Awarded National Agreement with Premier Inc. PMI is pleased to announce that it has been awarded a […] The post Progressive Medical, Inc. Awarded Drug Reconstitution and Transfer Devices Agreement with Premier, Inc. appeared first on Progressive Medical, Inc.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharma Marketing Network
MARCH 23, 2023
Pharmaceutical advertising is a crucial aspect of promoting and marketing drugs to the public. With the increase in competition, it has become essential for pharmaceutical companies to find the most effective methods of advertisement to reach their target audience. In this article, we will discuss the most effective types of advertisement in the pharmaceutical industry and provide examples and statistics to support our findings. 1.
Pharma Leaders
MARCH 23, 2023
Dewpoint Therapeutics has formed a research and development partnership with Novo Nordisk for identifying drug candidates to treat insulin resistance and diabetic complications. Dewpoint’s discovery platform related to biomolecular condensates will be used to identify the drug candidates. The collaboration will enable combination of Novo Nordisk’s expertise in treating diabetes and metabolic diseases and Dewpoint’s discovery and artificial intelligence (AI) technology platform to detect modulato

Pharma Marketing Network
MARCH 23, 2023
The Food and Drug Administration (FDA) has recently approved a new drug for the treatment of Alzheimer’s disease called Leqembi. This approval marks a major milestone in the fight against Alzheimer’s, which is the sixth leading cause of death in the United States and affects over 5 million people. Leqembi, developed by pharmaceutical company Biohaven, is an oral treatment that is designed to target the root cause of Alzheimer’s, which is the accumulation of a protein called bet
Pharmaceutical Technology
MARCH 23, 2023
China’s National Medical Products Administration (NMPA) has granted emergency use authorisation (EUA) for CSPC Pharmaceutical Group’s messenger RNA (mRNA) vaccine, SYS6006, to treat Covid-19. With this regulatory approval, CSPC Pharmaceutical is claimed to be the first company to receive approval for providing an mRNA vaccine in the country. The latest authorisation from the Chinese health authority comes as cases of Covid-19 decline after a recent surge.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Let's personalize your content