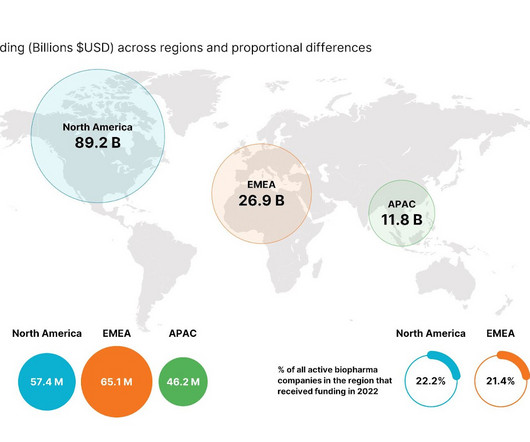
2024 forecast: M&A saw an uptick in 2023. Analysts expect the trend to continue
Fierce Pharma
DECEMBER 22, 2023
Even with the Federal Trade Commission keeping a watchful eye on the biopharma industry and the economic landscape giving some players pause, mergers and acquisitions are back on the rise. | Even with the Federal Trade Commission keeping a watchful eye on the biopharma industry and the economic landscape giving some players pause, mergers and acquisitions are back on the rise.




































Let's personalize your content