EU plots major pharma reforms—but industry is already pushing back
Fierce Pharma
APRIL 28, 2023
EU plots major pharma reforms—but industry is already pushing back zbecker Fri, 04/28/2023 - 18:04

Fierce Pharma
APRIL 28, 2023
EU plots major pharma reforms—but industry is already pushing back zbecker Fri, 04/28/2023 - 18:04

MedCity News
APRIL 28, 2023
Amazon recently announced that it is shuttering its Halo division, a line of wearable health and fitness devices. This marks the third time in about two years that the e-commerce giant has closed one its healthcare businesses — Amazon Care failed last year, and Haven failed in 2021.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
APRIL 28, 2023
With negative FDA panel vote, AstraZeneca may look at narrow Lynparza nod in prostate cancer aliu Fri, 04/28/2023 - 13:31

MedCity News
APRIL 28, 2023
In some ways, the Drug Enforcement Agency’s (DEA) proposed rules for prescribing controlled substances via telemedicine are a sign of progress.

Fierce Pharma
APRIL 28, 2023
Playing catch-up with Merck, Pfizer scores pediatric approval for Prevnar 20 kdunleavy Fri, 04/28/2023 - 07:11

PharmaTech
APRIL 28, 2023
Todd Andrews, global director of Applications and Business Development at CPC, discusses sterile aseptic connectors, flexibility in manufacturing, and more in an interview held at INTERPHEX 2023.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Pharmaceutical Technology
APRIL 28, 2023
The US Food and Drug Administration (FDA) has granted an Orphan Drug Designation to Editas Medicine’s gene therapy EDIT-301 in sickle cell disease, based on an April 27 announcement. The US agency previously granted the Orphan Drug Designation to EDIT-301 for its study in beta thalassemia, in May 2022. The company is studying EDT-301 in sickle cell disease in a Phase I/II RUBY study (NCT04853576), and is on track to provide a clinical update by mid-2023.

Fierce Pharma
APRIL 28, 2023
Otsuka, Lundbeck gain FDA nod for longer-acting version of schizophrenia drug Abilify kdunleavy Fri, 04/28/2023 - 10:29
MedCity News
APRIL 28, 2023
Merck, Sanofi, and Eli Lilly joined the Series A financing of Therini Bio, a startup developing a drug that selectively targets fibrin as a way of reducing disease-driving inflammation in neurodegeneration and eye disorders such as diabetic macular edema. Therini is preparing to advance its lead program to clinical testing.

Fierce Pharma
APRIL 28, 2023
The first Humira biosim is doing Amgen more harm than good—for now aliu Fri, 04/28/2023 - 09:52

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
APRIL 28, 2023
The Rockefeller Foundation is funding two produce prescription pilot programs at VA healthcare systems in Salt Lake City, Utah, and Houston, Texas. Eligible veterans will be able to enroll in About Fresh’s Fresh Connect program, which provides $100 per month through a debit card, allowing the veterans to buy healthy foods at the grocery store.

European Pharmaceutical Review
APRIL 28, 2023
NEW, FLEXIBLE, data-driven manufacturing models are emerging to address the pharmaceutical industry’s need for greater operational efficiency. While new approaches aim to solve the unique challenges of the sector, like adhering to strict compliance regulations, digitalisation has quickly developed into a pathway for achieving information technology (IT)/operational technology (OT) integration, edge-to-enterprise connectivity, and overall operational excellence.

MedCity News
APRIL 28, 2023
Too often today, BIPOC communities must deal with mental health obstacles without the necessary education, support, and coping strategies. Fortunately, healthcare organizations have opportunities to start addressing these challenges by building cultural competency and by strengthening community education, trust, and access.

European Pharmaceutical Review
APRIL 28, 2023
A new global research consortium is being established to address the global impact of antimicrobial resistance (AMR). Centres for Antimicrobial Optimisation Network (CAMO-Net) , brings together research teams from the University of Liverpool and Imperial College London in the UK, the University of Cape Town in South Africa, the Infectious Diseases Institute in Uganda and the Faculty of Medicine at the University of São Paulo in Brazil. ”… this network… represents a major commitment t

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

MedCity News
APRIL 28, 2023
Ask the Investor sessions at MedCity INVEST at the Ritz Carlton in Chicago May 22-24 offer opportunities for startups to talk with investors in intimate settings. Spots are limited so register today!
European Pharmaceutical Review
APRIL 28, 2023
The European Commission ’s (EC’s) reform of the EU pharmaceutical legislation published on 26 April 2023, adopted a proposal for a new Directive and a new Regulation. These amend and replace the existing general pharmaceutical legislation. According to the EC, the revision is intended to: Ensure all EU patients can access safe, effective, and affordable medicines Enhance the security of medicine supply and availability, regardless of where patients live in the EU Continue to make Europe an attra

PharmaVoice
APRIL 28, 2023
An industry veteran, innovator and curator, Dr. Amir Kalali’s vision for the future is patient-focused.

Copyright Clearance Center
APRIL 28, 2023
The post Publishing Opens Its Salary Wallet appeared first on Copyright Clearance Center.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

PharmaTimes
APRIL 28, 2023
Therapy concerns the treatment of adults with axial spondyloarthritis and psoriatic arthritis

Pharmaceutical Technology
APRIL 28, 2023
The US Food and Drug Administration (FDA) has approved Pfizer’s 20-valent pneumococcal conjugate vaccine, PREVNAR 20 , to prevent invasive pneumococcal disease (IPD) in infants and children aged six weeks to 17 years. PREVNAR 20 has been designed to avert IPD caused by 20 streptococcus pneumoniae (pneumococcal) serotypes. It has also been approved by the FDA for otitis media prevention in infants and children aged between six weeks and five years caused by the original seven serotypes contained

PharmaTimes
APRIL 28, 2023
Kite’s two CAR T-Cell therapies involve treating several different types of blood cancer

PharmaTech
APRIL 28, 2023
Sarah Stevens, senior vice-president and head of Drug Product Development & Manufacturing at Quotient Sciences, discusses various facets of the modern outsourcing industry.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Clarify Health
APRIL 28, 2023
Unlocking value from analytics—do you have the right fuel? Data and analytics are driving healthcare organizations forward. However, with rising economic pressure, IT and data science teams spread thin, and many health systems suffering losses this year, CIOs and data and analytics leaders need to do more with less. Building a growth and value strategy at scale, with speed and precision, requires big data efficiencies, and the ability to draw upon billions of patient journeys.

Penrod
APRIL 28, 2023
Here at Penrod, Kevin helps plan, create, maintain, improve, and deliver reusable solutions for sought-after Healthcare use cases. “My current role is to transform these solutions into reusable assets for any Healthcare project,” he says. On a weekly basis, he stays busy by seeking out Delivery team members with availability to collaborate on creating and improving our reusable solution library.

Pharmaceutical Technology
APRIL 28, 2023
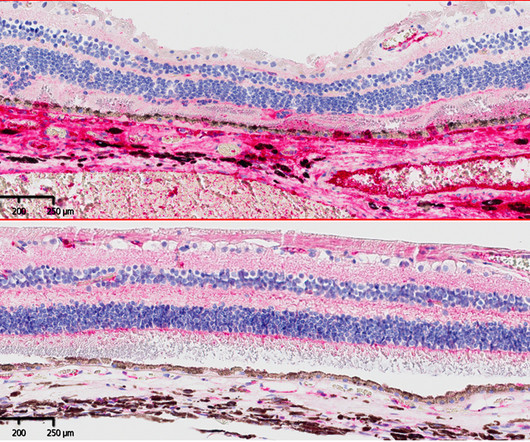
Ocugen has received orphan drug designation from the US Food and Drug Administration for its OCU410ST (AAV5-hRORA) to treat ABCA4 – linked retinopathies. OCU410ST is an adeno-associated virus (AAV) serotype 5 capsid protein, which comprises a gene construct that encodes human retinoic acid receptor (RAR)-related orphan receptor alpha. It is intended to treat ABCA4-linked retinopathies including retinitis pigmentosa 19 (RP19), Stargardt disease and cone-rod dystrophy 3 (CORD3).

Contrarian Sales Techniques
APRIL 28, 2023
Discovering Your Inner Compass Let’s dive into the fascinating world of nurturing self-awareness. You might be thinking, "Why should I even care about self-awareness?" Well, my friend, self-awareness is the cornerstone of personal growth, and it's an essential ingredient for living a more intentional, fulfilling life. Let's explore this together, shall we?

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
Pharmaceutical Technology
APRIL 28, 2023
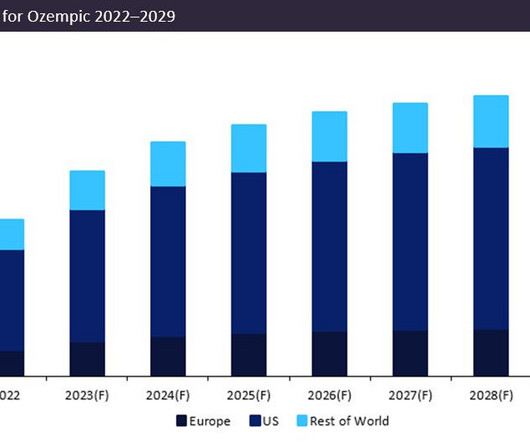
Novo Nordisk’s leading drug Ozempic (semaglutide) is forecast to demonstrate a sales growth of 23% in 2023. Ozempic’s forecast 2023 sales of $12.5bn consolidate its position as the dominant market leader, with projected sales in 2023 54% greater than closest competitor Trulicity (dulaglutide) by Eli Lilly, which anticipates sales of $8bn. Originally granted approval in its largest market, the US, in 2017, Ozempic has subsequently obtained approval for three distinct dosages, 0.5mg, 1.0mg, and 2.
Legacy MEDSearch
APRIL 28, 2023
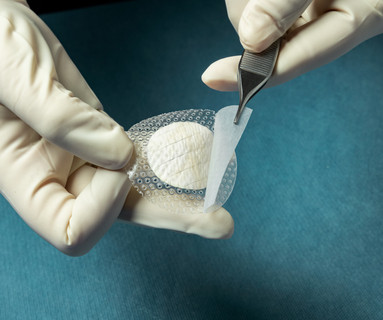
Kerecis ® today announced MariGen ® Shield, which integrates the company’s proven fish-skin graft with a silicone contact layer for treating chronic and complex wounds. The medical-fish-skin company also announced the results of a clinical study comparing the effectiveness of the Kerecis fish-skin grafts to a standard of care for diabetic foot ulcers.
Pharmaceutical Technology
APRIL 28, 2023
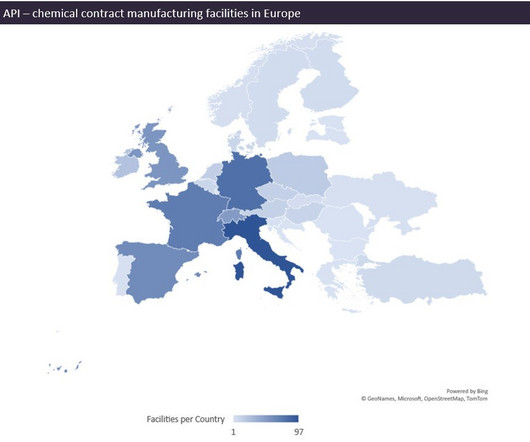
An EU study claims that building more API facilities in Central and Eastern Europe could help solve drug shortages and weaknesses in the supply chain. The literature review suggests the Czech Republic, Poland, and Hungary as sites for new production locations in Europe given that their staff costs are lower than in Western Europe, but notes that higher-income countries, such as Germany, the Netherlands, and Belgium also make attractive production locations.

Pharmatutor
APRIL 28, 2023
Expert Committee on Pharmacy Education, Law and Regulator admin Fri, 04/28/2023 - 15:45 ABOUT AUTHOR Dr. R. S. Thakur Chief Editor, Journal of Pharmaceutical Research Krupanidhi College of Pharmacy, Bengaluru, India. Email : drramsthakur@gmail.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content