Johnson & Johnson cites rebates and discounts for drug price increases
Fierce Pharma
MAY 26, 2023
Johnson & Johnson cites rebates and discounts for drug price increases kdunleavy Fri, 05/26/2023 - 07:36

Fierce Pharma
MAY 26, 2023
Johnson & Johnson cites rebates and discounts for drug price increases kdunleavy Fri, 05/26/2023 - 07:36

Nixon Gwilt Law
MAY 26, 2023
As a law firm focused specifically on healthcare innovation, we get really excited about developing areas of the healthcare industry. One area we are particularly excited about? Virtual Reality. Providers are already integrating Virtual Reality ( VR ) into patient care in a variety of ways. For example, using VR to gamify physical therapy exercises for pediatric patients to keep them engaged in their treatment, or to provide targeted exposure therapy in a controlled environment for patients with
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 26, 2023
EU regulators recommend yanking authorization for Novartis' sickle cell med Adakveo after phase 3 miss zbecker Fri, 05/26/2023 - 10:48

MedCity News
MAY 26, 2023
Although there is uncertainty and risk, the implementation of AI with the right compliance framework and infrastructure offers an exciting opportunity to transform healthcare into a new frontier with improved patient outcomes and increased efficiency.
Fierce Pharma
MAY 26, 2023
While Pfizer, BioNTech agree to cut COVID vaccine supply to Europe, Moderna sets up shop in China kdunleavy Fri, 05/26/2023 - 10:03
Pharmaceutical Technology
MAY 26, 2023
The US Food and Drug Administration (FDA) has granted priority review for Takeda and HUTCHMED’s new drug application (NDA) for fruquintinib. Fruquintinib is a selective and potent oral VEGFR -1, -2 and -3 receptors inhibitor and is used for the treatment of adults with previously treated metastatic colorectal cancer. Fruquintinib will be the first and only highly selective inhibitor of these receptors approved in the US.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Pharmaceutical Technology
MAY 26, 2023
The immuno-oncology market continues to evolve as treatments establish their presence across different types of cancer, having seen approvals in multiple indications in the past decade. While the market was worth over $6 billion in 2012, this has now grown to nearly $48 billion only a decade later, said Avigayil Chalk, PhD, GlobalData’s Senior Oncology and Haematology analyst, at an immuno-oncology webinar held on May 23.
Fierce Pharma
MAY 26, 2023
Fierce Pharma Asia—Carvykti's EU filing; Thermo Fisher's Singapore expansion; Celltrion's Humira biosim nod aliu Fri, 05/26/2023 - 08:43

Pharmaceutical Technology
MAY 26, 2023
Krystal Biotech has received approval from the US Food and Drug Administration for topical gene therapy VYJUVEK to treat dystrophic epidermolysis bullosa (DEB) in adults and in children aged six months and above. VYJUVEK is designed to address the underlying genetic cause of the disease. An excipient gel applied topically is a key component of the therapy, which is supplied by Krystal’s client, Berkshire Sterile Manufacturing (BSM).

Fierce Pharma
MAY 26, 2023
AstraZeneca adds endometrial cancer to Imfinzi-Lynparza combo's positive trial readouts aliu Fri, 05/26/2023 - 10:32

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

European Pharmaceutical Review
MAY 26, 2023
The UK Chancellor has announced a £650 million life sciences growth package (Life Sci for Growth) to help deliver the Science and Technology framework through reforming regulation, boosting investment and driving up talent and skills. Bringing together 10 different policies, the package includes: A total of £121 million to improve commercial clinical trials to bring new medicines to patients faster Up to £48 million of new money for scientific innovation to prepare for any future health emergenc
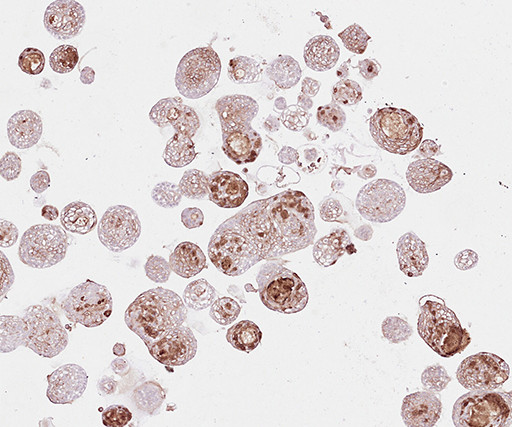
Medgadget
MAY 26, 2023
Researchers at the Hubrecht Institute in the Netherlands have developed a biobank of cancer organoids using tissue samples obtained from head and neck cancer patients. So far, the team used the biobank to validate tumor biomarkers. Excitingly, they also correlated patient treatment responses with organoid treatment responses, suggesting that the organoids provide a good proxy for testing new treatments and for designing a personalized treatment plan for individual patients.
European Pharmaceutical Review
MAY 26, 2023
The Medicines and Healthcare products Regulatory Agency ( MHRA ) has announced that new international regulatory recognition routes for medicines will be established using approvals from the EU, Switzerland, US, Canada, Australia, Singapore and Japan. According to the MHRA, the recognition routes mark the start of a new international recognition framework for medicines that will be in place by the first quarter of 2024.

MedCity News
MAY 26, 2023
Medicare is failing its beneficiaries by not treating obesity as a disease. Just like people with high blood pressure often need to take medications to avoid disease-related complications, people with obesity need access to all appropriate, evidence-based therapies to combat the disease.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

PharmaVoice
MAY 26, 2023
Executives honored by the Healthcare Businesswomen’s Association share the key traits that help them drive results.

MedCity News
MAY 26, 2023
The Substance Abuse and Mental Health Services Administration identifies three core crisis services that should be available to those in need of behavioral health support: crisis hotlines, mobile crisis units and crisis stabilization. However, 33 state Medicaid programs don’t cover all of these services, a new analysis by the Kaiser Family Foundation found.

PharmaTimes
MAY 26, 2023
Life Sciences Council meeting hosted by chancellor Jeremy Hunt has unfolded in Downing Street

MedCity News
MAY 26, 2023
A conversation with Suchi Saria, associate professor of medicine at John Hopkins University and director of its Machine Learning and Healthcare Lab about responsible AI.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

PharmaTimes
MAY 26, 2023
Therapy has been developed for use among children and adolescents with growth hormone deficiency

MedCity News
MAY 26, 2023
Hospitals are places of healing, but they have also become sites of gun violence. A panel at the MedCity News INVEST conference discussed the ways hospitals can reduce threats and de-escalate situations before they become violent.

PharmExec
MAY 26, 2023
Ahead of ASCO and BIO, Michael Bailey CEO of AVEO Oncology, discusses the company becoming a LG Chem subsidiary and the future for drug development in the combined entity.

MedCity News
MAY 26, 2023
Presented by the Consulate General of Canada, the Canadian healthcare startup showcase included five companies. Technologies included a patient transfer solution, care coordination, women’s health, exercise tracking and remote monitoring for dysphagia, and mental health support.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
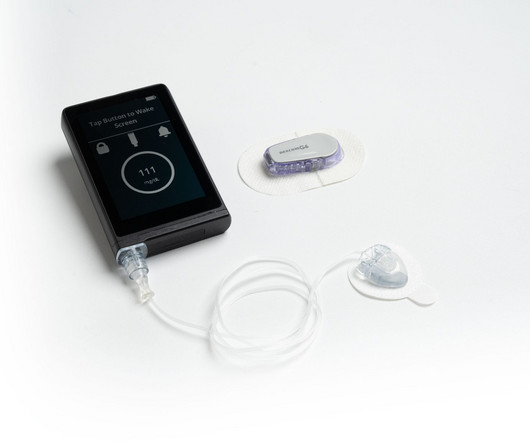
Pharmaceutical Technology
MAY 26, 2023
Chronic disease tech company Convatec Group has partnered with Beta Bionics to manufacture the insulin delivery system iLet Bionic Pancrease. According to Beta Bionics, this is the only first and only automated insulin delivery system that carries 100% of doses and does not require carb counting. The device received US Food and Drug Administration 510(k) clearance last week for those ages six years and above living with type 1 diabetes.

PharmaTech
MAY 26, 2023
Challenges to approval decisions have prompted FDA officials to reexamine of the role and composition of the agency’s many advisory committees and to explore options for change.
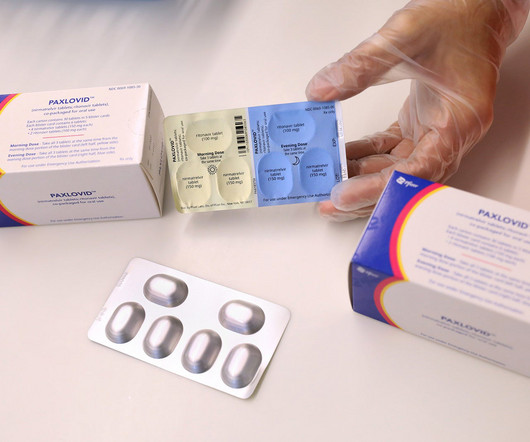
Pharmaceutical Technology
MAY 26, 2023
After earning multimillion dollar revenues while being an authorised preferred treatment for Covid-19, the US Food and Drug Administration (FDA) has granted a full approval to Pfizer’s oral antiviral Paxlovid (nirmatrelvir + ritonavir). The label is specified for the treatment of “mild-to-moderate Covid-19 in adults who are at high risk for progression to severe COVID-19, including hospitalisation or death.

PharmaTech
MAY 26, 2023
Myrna Wilson, director, Global Marketing, Strategic Growth and Technical Sales, Pharmaceutical Ingredients, Univar Solutions, discusses the state of the biopharma supply chain, movements in the patent industry, and more.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Legacy MEDSearch
MAY 26, 2023
The American Thoracic Society International Conference (ATS 2023) took place in Washington, D.C. from 19 to 24 May 2023. During the conference on 23 May, French MedTech Biosency unveiled its latest clinical results demonstrating the ability of its BVS 3 technology to predict acute COPD (chronic obstructive pulmonary disease) exacerbations on average 3 days prior to hospitalisation in 86% of cases, with only 9% false positives.

Clarify Health
MAY 26, 2023
The transformative capacity of data analytics is shaping the future of healthcare, driving new levels of efficiency, effectiveness, and patient-centric care. Network strategists at leading health plans have a front seat in witnessing first-hand the profound impact that healthcare data analytics can have on improving the daunting task of health plan network design.
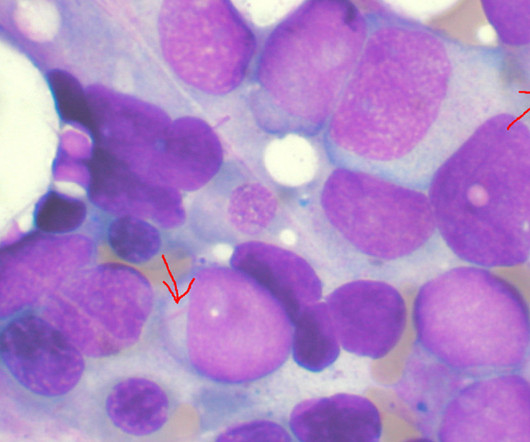
Pharmaceutical Technology
MAY 26, 2023
Daiichi Sankyo has received approval from Japan’s ministry of health, labour and welfare (MHLW) for VANFLYTA (quizartinib) to treat acute myeloid leukaemia (AML) patients who are positive for the FLT3-ITD mutation. The approval is based on the results of the placebo-controlled, randomised, double-blind, global Phase III QuANTUM-First trial of VANFLYTA in combination with standard cytarabine and anthracycline induction.

Pharma Leaders
MAY 26, 2023
Biotechnology company VarmX has raised an additional €30m in a Series B2 financing round to obtain investigational new drug (IND) approval for its lead compound VMX-C001. VMX-C001 is a modified recombinant human blood clotting factor X that enables patients taking direct oral anticoagulant blood thinners to undergo emergency surgery without the risk of bleeding.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content