Quitting Lilly's obesity drug tirzepatide may be difficult for many patients, exec says
Fierce Pharma
APRIL 27, 2023
Quitting Lilly's obesity drug tirzepatide may be difficult for many patients, exec says kdunleavy Thu, 04/27/2023 - 15:08

Fierce Pharma
APRIL 27, 2023
Quitting Lilly's obesity drug tirzepatide may be difficult for many patients, exec says kdunleavy Thu, 04/27/2023 - 15:08

MedCity News
APRIL 27, 2023
While the digital health sector might feel a little cramped in some areas, there are wide open spaces in others — and investors are ready to funnel money into companies looking to innovate these lesser-discussed aspects of care delivery and healthcare technology. Some of these areas include Medicaid, generative AI and patient-to-patient support.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
APRIL 27, 2023
Pascal Soriot deflects shareholder discord over AstraZeneca's legal battles amid 'very encouraging' start to the year fkansteiner Thu, 04/27/2023 - 09:39

MedCity News
APRIL 27, 2023
Taking inspiration from one of the most influential women in modern medicine, Elizabeth Blackwell, who was the first American woman to earn a medical degree, we must persist – with optimism, courage and a fearless commitment to create a more equitable future for all women.

Fierce Pharma
APRIL 27, 2023
Bristol's multiple new launches won't stop it from scouting for M&A deals, CEO says aliu Thu, 04/27/2023 - 10:16

MedCity News
APRIL 27, 2023
About 8% of Black adults and 7% of Hispanic adults skipped care because of transportation challenges in the last year, compared to 4% of White adults and 2% of Asian adults, according to the Robert Wood Johnson Foundation. In addition, 14% of adults with low family incomes passed on care.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Clarivate
APRIL 27, 2023
Our latest Global Research Report from the Institute for Scientific Information (ISI) , “U.S. research trends: The impact of globalization and collaboration,” raises important questions about how well past investment has prepared the U.S. scientific enterprise to achieve its goals. Our findings suggest that while the U.S. remains a leading science and technology power, it must acknowledge its shrinking domestic research capacity and work collaboratively with resourceful competitors to maintain i

Fierce Pharma
APRIL 27, 2023
After recent M&A moves, Merck CEO says he's thinking beyond Keytruda's eventual downfall kdunleavy Thu, 04/27/2023 - 12:50

Pharmaceutical Technology
APRIL 27, 2023
The European Commission’s (EC) long-anticipated pharma reform plans in the European Union have finally been unveiled , indicating a focus on improving access to medicines across the bloc while cutting down on market exclusivity. Described as the largest reform in over 20 years, the proposed revision touches on multiple topics ranging from unequal access to innovative medicines across the EU to new environmental protections.

Fierce Pharma
APRIL 27, 2023
As Humira biosims take hold, longtime AbbVie CEO Richard Gonzalez talks succession plans zbecker Thu, 04/27/2023 - 12:30

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
MedCity News
APRIL 27, 2023
Quest Diagnostics is acquiring liquid biopsy company Haystack Oncology. The startup’s technology is used for identifying cancer patients who need adjuvant therapy.
Fierce Pharma
APRIL 27, 2023
Fierce Pharma Asia—Daiichi's leukemia delay, Enhertu gains; Glenmark's settlement aliu Thu, 04/27/2023 - 22:47

MedCity News
APRIL 27, 2023
Through a new partnership, Hopper Health’s providers and peer navigators will receive a personalized training plan from Violet that will help the clinicians and navigators understand how to provide inclusive care for patients of color and LGBTQ+ communities. It will teach topics including anti-racism, allyship, social determinants of health, trauma-informed care and other areas.

Fierce Pharma
APRIL 27, 2023
'The Top Line': A special series on Narcan, the life-saving nasal spray tcarey Thu, 04/27/2023 - 15:30

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

MedCity News
APRIL 27, 2023
CMS recently fined two hospitals for alleged violations of its price transparency rule. These fines are only the third and fourth penalties issued by the agency since the rule took effect on January 1, 2021. These fines may become more common — CMS also announced that it is updating its enforcement process for the rule.
European Pharmaceutical Review
APRIL 27, 2023
Immunotherapy treatment blinatumomab will now become standard treatment worldwide for babies with acute lymphoblastic leukaemia (ALL). This decision is based on new results from an international trial published in the New England Journal of Medicine. Blinatumomab significantly improved survival, with the rate increasing from 66 percent to 93 percent, compared to individuals just given prior chemotherapy.
MedCity News
APRIL 27, 2023
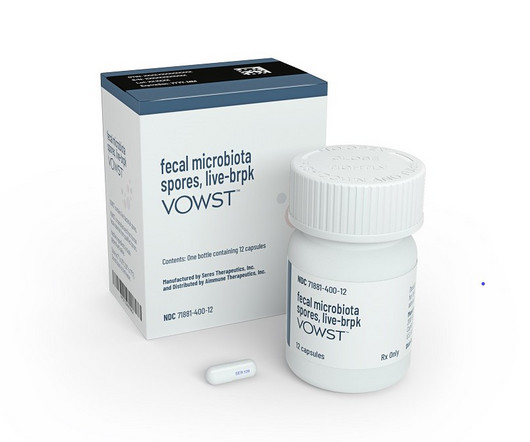
Recurrent Clostridioides difficile infection can be treated with fecal microbiota transplants. FDA approval of Seres Therapeutics’ Vowst makes it the first oral microbiome therapy.
Pharmaceutical Technology
APRIL 27, 2023
Seres Therapeutics and Nestlé Health Science have received approval from the US Food and Drug Administration (FDA) for Vowst (faecal microbiota spores, live-brpk) for preventing the recurrence of C difficile infection (CDI) in adults. Vowst is an orally administered microbiota-based therapeutic, previously known as SER-109, and is indicated for the prevention of recurrence of CDI in people aged 18 years and above following an antibacterial treatment for recurrent CDI (rCDI).

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
European Pharmaceutical Review
APRIL 27, 2023
Through pre-clinical and clinical trial success, adeno-associated viral vectors (AAVs) have consistently demonstrated their effectiveness as a tool for gene delivery to treat a variety of human diseases. As we stand at the precipice of an era that truly embraces gene therapies, there are clearly still a number of challenges to overcome to fully realise the potential of innovative AAVs.

PharmaTech
APRIL 27, 2023
Yaakov Nahmias, founder and chief scientific officer of Tissue Dynamics, discusses the barriers in implementing organ on a chip technology.

European Pharmaceutical Review
APRIL 27, 2023
In recent years, the biopharmaceutical industry has found itself at a crossroads – being able to offer advanced and truly innovative treatments, such as gene therapies, but facing the complex challenge of creating manufacturing standards for the biotechnology sector from the ground up. Solving this issue will improve industry best practice, increase public confidence and create a more efficient pathway to patients; but it necessitates enhanced collaboration between companies.
PharmExec
APRIL 27, 2023
While ESG is still in the early stages for pharma, many companies are moving forward with a need for compliance.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

European Pharmaceutical Review
APRIL 27, 2023
[the CHMP’s recommendation of Cosentyx] brings us one step closer to offering the first new hidradenitis suppurativa (HS) treatment in nearly a decade” The Committee for Medicinal Products for Human Use (CHMP) has granted a positive opinion for biologic Cosentyx ® (secukinumab). The recommendation for a marketing authorisation “brings us one step closer to offering the first new hidradenitis suppurativa (HS) treatment in nearly a decade,” commented Marie-France Tschudin, President of Novartis I

Copyright Clearance Center
APRIL 27, 2023
The post Workflow of the Future: Sustainable Business Models Webcast appeared first on Copyright Clearance Center.

European Pharmaceutical Review
APRIL 27, 2023
Christopher Boerner, PhD, Executive Vice President (EVP), Chief Commercialization Officer of Bristol Myers Squibb will become the company’s new CEO on November 1 2023. Effective immediately, Boerner has been named its EVP, Chief Operating Officer. The pharma company’s current CEO and Chairman of the Board Dr Giovanni Caforio will retire on November 1 2023.

Pharmaceutical Commerce
APRIL 27, 2023
From logistics lessons learned, how pharma can navigate new opportunities surfacing in ocean shipping.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

PharmaTimes
APRIL 27, 2023
Duo will collaborate to develop pluripotent stem cell-based microfluidic cultures for pain research
Pharmaceutical Technology
APRIL 27, 2023
The UK’s National Institute for Health and Care Excellence (NICE) has recommended two new personalised immunotherapy therapies from Kite Pharma to treat aggressive forms of blood cancer for the Cancer Drugs Fund (CDF). Established in 2011, the CDF is a source of funding to increase patient access to cancer drugs in the UK. The recommended chimeric antigen receptor (CAR) T-cell therapies include Yescarta (Axicabtagene ciloleucel) and Tecartus (Brexucabtagene autoleucel).

PharmaTimes
APRIL 27, 2023
Therapy is being used during pivotal phase 1 study into patients with pancreatic cancer

Clarify Health
APRIL 27, 2023
In this 4-minute video, Jayson Harpster , Product Director for Clarify’s provider incentive software and program, discusses how payers can influence practice patterns and drive provider behavior change inside busy medical practices. Watch to learn three critical components of designing an effective provider incentive program. Transcript: In health care, we often have tons of data with no clear way to use it to drive real change.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content