Vaxess' flu vaccine patch passes early clinical test, clearing path for further development
Fierce Pharma
DECEMBER 20, 2022
Vaxess' flu vaccine patch passes early clinical test, clearing path for further development. Tue, 12/20/2022 - 04:57.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical flu
clinical flu 
Fierce Pharma
DECEMBER 20, 2022
Vaxess' flu vaccine patch passes early clinical test, clearing path for further development. Tue, 12/20/2022 - 04:57.

European Pharmaceutical Review
NOVEMBER 29, 2022
million, UK-wide adaptive platform trial (APT) has been launched to find effective treatments for people hospitalised with severe flu for the first time, testing multiple options simultaneously in thousands of people then modifying treatments quickly. National Institute for Health and Care Research (NIHR)’s Clinical Research Network.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
pharmaphorum
NOVEMBER 25, 2022
While the shot is still in preclinical development, if it works in human trials it could raise the prospect of eventually sidestepping the annual scramble to guess the most likely strains to be circulating in the following flu season, and protect against future flu pandemics.
European Pharmaceutical Review
MARCH 26, 2024
. “mRNA-1283 is a critical component of our combination vaccine against flu and COVID-19, mRNA-1083, and this milestone gives us confidence in our ability to bring this much needed vaccine to market.” 5 and original virus strains of SARS-CoV-2, compared to Moderna’s Spikevax vaccine.

Pharmaceutical Technology
DECEMBER 20, 2023
FLU-SV-mRNA is under clinical development by Curevac and currently in Phase I for Influenza A Virus, H1N1 Subtype Infections.

Pharmaceutical Technology
DECEMBER 20, 2023
FLU-SV-mRNA is under clinical development by Curevac and currently in Phase I for Influenza A Virus, H1N1 Subtype Infections.

pharmaphorum
DECEMBER 22, 2022
It also said the alliance will see Moderna invest in R&D in the UK over the next 10 years, including running a number of clinical trials and providing grant funding to UK universities, while the manufacturing facility will create more than 150 highly skilled jobs. The first vaccines are due to be produced at the new facility in 2025.

European Pharmaceutical Review
DECEMBER 22, 2022
It also has potential to develop vaccines for other respiratory diseases, such as flu and respiratory syncytial virus. This will include running a significant number of clinical trials in the UK. The Moderna Innovation and Technology Centre (MITC) is intended to provide access to a UK-made supply of COVID-19 jabs.

World of DTC Marketing
APRIL 11, 2021
Further scientific research into the virus and recognition that it was not like influenza A, a flu virus known to cause bronchitis, would transpire over the next 30 years. . Plus, clinical trial settings aren’t always the same as real-world settings. Plus, clinical trial settings aren’t always the same as real-world settings.
European Pharmaceutical Review
NOVEMBER 23, 2022
Clinical development of Hemgenix was led by uniQure. Sponsorship of the clinical trials transitioned to Australian biopharma company CSL after it acquired global rights to commercialise the treatment in May 2021. Haemophilia B clinical trial. —the possibility of freedom from the need for regular, ongoing infusions.”.
Pharmaceutical Technology
JANUARY 6, 2023
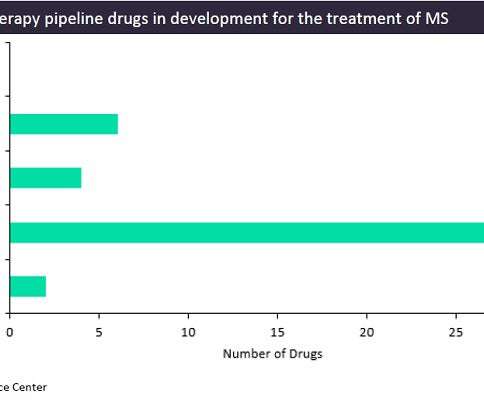
Currently, there is a range of cell therapies and gene-modified cell therapies in development, from the discovery stage to Phase II clinical trials, with six different cell therapies in Phase II development.
pharmaphorum
SEPTEMBER 22, 2022
Le Vert says Osivax’s technology differs, however, as it attacks the virus’ T-cells, making the vaccine more effective in the long term, and that clinical trials have shown promise. The CDC analyses the effectiveness of flu vaccines yearly and has done so since the 2003/2004 flu season.
pharmaphorum
SEPTEMBER 6, 2022
1-adapted bivalent vaccine was clinically shown to have a favourable safety profile with immunogenicity against both wild type and Omicron strains”. Data from a clinical trial found side effects to typically be mild and self-resolving and to be no worse than the original booster dose.

pharmaphorum
DECEMBER 15, 2022
2 Symptoms including congested or runny nose, coughing, fever, and sore throat mean that RSV can easily be mistaken for other respiratory viruses which are also prevalent during the same season, such as COVID-19 or flu. Mayo Clinic. RSV is one of the most common respiratory viruses, causing coughs and colds during the winter months.

Pharmaceutical Technology
JULY 18, 2022
In clinical trials, the adjuvanted formulation induced the necessary immune response using a reduced amount of antigen versus a formulation without adjuvant, indicating that more doses could be available during a pandemic. H1N1, also called Swine Flu is the latest influenza pandemic in the country.

European Pharmaceutical Review
OCTOBER 17, 2022
Benefits and safety parameters of the vaccine were evaluated in a total of 19 clinical trials using 27,000 participants between 15 months and 60 years old. Dengue is a mosquito-borne illness caused by four types of the dengue virus that usually causes mild, flu-like symptoms. About dengue.

pharmaphorum
OCTOBER 12, 2022
“Continuing our strategic alliance with Merck is an important milestone as we continue to grow our mRNA platform with promising clinical programmes in multiple therapeutic areas.” The post Merck gets on board with Moderna cancer vaccine in $250m deal appeared first on.
European Pharmaceutical Review
AUGUST 25, 2022
Now, clinical trials are assessing an allogeneic therapy for glioblastoma called NeuroNova, which maximises the power of an oncolytic virus by hiding it from the patient’s immune system within stem cells. This persistent roadblock prevented these foundational studies from translating to the clinic. NeuroNova in action.
pharmaphorum
OCTOBER 17, 2022
Most people exhibit mild, flu-like symptoms, but some patients develop severe disease, including potentially fatal bleeding and organ damage. The likelihood for severe disease increases with repeated dengue infection.

PM360
DECEMBER 20, 2023
The out-of-pocket (OOP) percentage for the same service is lowest in community clinics and highest in general hospitals. In most cases, imported innovative drugs cannot apply for local regulatory approval with global multicenter clinical trial results and a Chinese phase 3 clinical trial is mandatory.

Clarify Health
NOVEMBER 15, 2023
Using predictive models and trend data can help hospitals forecast patient flow so they can better allocate finite resources like clinical staff, hospital beds, and medical equipment. Models can analyze health trend data to help providers keep ahead of disease outbreaks or plan for seasonal health issues like the flu and RSV.

pharmaphorum
JANUARY 17, 2023
The UK government has one eye on the short-term, securing a supply of COVID-19 vaccines, but also towards future potential vaccines against the flu or other infectious diseases. In addition, the construction of a specialised manufacturing facility for mRNA vaccines was agreed.
PM360
JUNE 29, 2022
In December 2020, RB launched the Cough, Cold, and Flu Navigator with WebMD. The retailer has been gradually building out its network of in-store clinics—a bricks-and-mortar presence that will be complemented by its virtual care offering. Data can also play a role in connecting products to meet consumers’ needs in real-time.
pharmaphorum
SEPTEMBER 15, 2022
The 25,000-subject study is testing a nucleoside-modified mRNA (modRNA), quadrivalent vaccine that is targeted against four strains of the flu virus that the World Health Organization (WHO) expects will be circulating during the coming 2022/23 flu season in the northern hemisphere.

PM360
JULY 13, 2023
Second, a patient may be on the right regimen, in which their five or more drugs are prescribed based on clinical evidence. 15 Pharmacists are true experts in the clinical use of medications and see patients with chronic conditions up to 10 times more per year than their physicians do. different doctors during their lives.
Healthcare Success
JUNE 12, 2023
Host events or other meetups Consider hosting open houses, grand openings, flu vaccine clinics, or other informal meetups to generate buzz around your local businesses and build links in the process. Develop a strong local SEO linking strategy Local SEO linking is a must-have piece of your comprehensive SEO strategy.

Medico Reach
FEBRUARY 17, 2023
While the second helps you connect with licensed professional counselors, family and marriage therapists, clinical social workers, instantly. Customers can easily make an appointment when necessary to obtain treatment virtually quickly for common problems and conditions like Flu, Minor rashes, pink eye, allergies, etc.

PM360
NOVEMBER 21, 2022
In addition to the COVID-19 vaccine, most pharmacists are licensed to administer a wide range of vaccines and immunizations to protect against HPV, hepatitis, flu, and more. Messaging from the CDC and state and local government departments of health included a direct call to action—go to a pharmacy to get vaccinated.

European Pharmaceutical Review
SEPTEMBER 22, 2023
Benefits of the new RNA Centre of Excellence Importantly, CPI’s new facility is the first in the UK available to support “complete clinical manufacturing chain for RNA including lipid nanoparticle (LNP) production” Head of the RNA Centre of Excellence, Julie Anderson explained to EPR.

Legacy MEDSearch
JANUARY 3, 2023
The Visby Medical Respiratory Health Test is a fast, polymerase chain reaction (PCR) device that detects and differentiates between upper respiratory infections caused by Influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). .
Pharmaceutical Technology
JUNE 16, 2023
The developer has tested OVX836 in four completed clinical trials. The company aims to enrol more than 500 participants aged 18-60 across multiple clinical sites in Australia. Osivax’s OVX836 is joined by fellow T-cell vaccine Flu-v from Imutex as candidates already in Phase II trials.

Evolve Your Success
JANUARY 2, 2024
She moved from the clinical side to the sales side of the operating room back in 2014 and joined a major device company. Before I took this role a few months ago, I was the national manager for a field clinical team for a peripheral nerve StimRouter. I managed the team across the country of field clinical managers. Talk to us.

Pharmaceutical Technology
NOVEMBER 23, 2022
Based on findings from the multinational, open-label, single-arm Phase III HOPE-B clinical trial underway, the regulatory agency granted the approval. A rise in liver enzymes, headache, increased blood enzyme level and flu-like symptoms among others were observed to be the most frequent side effects of the gene therapy.

Pharmaceutical Technology
SEPTEMBER 10, 2008
Before the inception of this institute, the facility was oriented towards pre-clinical toxicology and breeding of the laboratory animals. ” New projects we have been involved with have helped the company to accelerate its development in the field of pre-clinical research. It will most probably lead to a new anti-flu vaccine.

World of DTC Marketing
MARCH 25, 2021
You might end up with a thing like the flu where every year, every two years, you need a boost,” Bancel told Forbes. Additional factors, such as the need for booster shots, present “a significant opportunity for our vaccine from a demand perspective, from a pricing perspective, given the clinical profile of our vaccine.”.

pharmaphorum
JANUARY 5, 2023
The biotech industry is starting to see real progress with AI-focused drug discovery companies reaching meaningful milestones or readouts of clinical trials – Relay Therapeutics and Recursion are two examples. AI has unintended consequences on biotech, and that’s a good thing.

European Pharmaceutical Review
JANUARY 16, 2023
Perhaps we will see a universal flu vaccine in the near future. Molecular Therapy – Methods & Clinical Development. The previous record for vaccine development and approval was four years for the measles vaccine. What were the most exciting innovations in pharmaceutical microbiology in 2022? Available from: [link].
Pharmaceutical Technology
JULY 1, 2022
Instead, it has stated that there is no clinical use of the drug in the prevention or treatment of Covid-19, the article detailed. Ian M Mackay’s tweet on rising flu cases in Australia. In addition, doctors who have prescribed the drug for Covid-19 are also expected to report the outcomes of the treatment to SAHPRA. Likes: 207.

Clarify Health
MARCH 15, 2023
Clinical cohorts. If they can see how their clinical cohort for cardiology overall is performing and what the opportunity is there … or orthopedics or diabetes management … if they have a good view into a particular clinical cohort, it makes for a really robust conversation for both. So we assign them. Keith: Yeah.

World of DTC Marketing
OCTOBER 16, 2020
KEY PONTIFICATION: If the coronavirus vaccine is 75 percent effective—which would be excellent, considering that the flu shot is only about 50 percent effective—roughly two-thirds of the population would need to be vaccinated, according to Paul Offit, the director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
European Pharmaceutical Review
SEPTEMBER 12, 2022
The virus usually causes painful skin lesions and flu-like symptoms. A clinical trial evaluating alternative strategies for administering Bavarian Nordic’s Jynneos monkeypox vaccine to increase the number of available doses has begun enrolling adult volunteers (aged 18 to 50 years). Intradermal vaccine delivery.

Pharma Marketing Network
JUNE 8, 2020
You better get people to a Virginia Mason or a Mayo Clinic or things like that. I mean Mayo Clinic, they found about 20% misdiagnosis. So, of course, if you don’t have the right diagnosis, everything is kind of harmful both medically and financially and so that’s where you got to have very specific strategies.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content